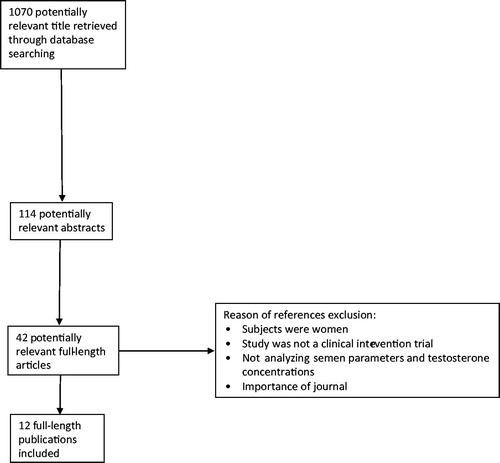
Figures & data
Figure 2. The actions of zinc regarding functional and structural improvements of the spermatozoa (Adapted from Hidiroglou and Knipfel [Citation11]. Zinc acts on the head (1), midpiece (2) and tail (3) of the spermatozoa. In the head, zinc supports the stability of the membrane and chromatin, acting on the nucleus. In the midpiece, zinc supports the mitochondrial energy processes, enhancing substrates by increasing both ATP and lipid oxidation. Mediated by actions both in the midpiece and tail, zinc further supports fibrillar contraction, enhancing sperm motility. Furthermore, zinc is also essential for sperm morphology and integrity. Increasing zinc in the seminal fluid may further improve the functionality of the spermatozoa to interact with the Egg Cell. ATP: Adenosine triphosphate.
![Figure 2. The actions of zinc regarding functional and structural improvements of the spermatozoa (Adapted from Hidiroglou and Knipfel [Citation11]. Zinc acts on the head (1), midpiece (2) and tail (3) of the spermatozoa. In the head, zinc supports the stability of the membrane and chromatin, acting on the nucleus. In the midpiece, zinc supports the mitochondrial energy processes, enhancing substrates by increasing both ATP and lipid oxidation. Mediated by actions both in the midpiece and tail, zinc further supports fibrillar contraction, enhancing sperm motility. Furthermore, zinc is also essential for sperm morphology and integrity. Increasing zinc in the seminal fluid may further improve the functionality of the spermatozoa to interact with the Egg Cell. ATP: Adenosine triphosphate.](/cms/asset/afeaebfe-23db-4dcd-ad45-90a5f9a86f70/itam_a_1573220_f0002_b.jpg)
Figure 3. Zinc is important for the spermatogenesis process, acting in the seminiferous tubules. The spermatids are developed in the spermatozoa through Zinc-Containing Proteins and several complex enzymatic reactions. Adapted from Hidiroglou and Knipfel [Citation11].
![Figure 3. Zinc is important for the spermatogenesis process, acting in the seminiferous tubules. The spermatids are developed in the spermatozoa through Zinc-Containing Proteins and several complex enzymatic reactions. Adapted from Hidiroglou and Knipfel [Citation11].](/cms/asset/7503cf94-93e3-4fbe-934d-8f34c6320abd/itam_a_1573220_f0003_c.jpg)
Figure 4. Proposed hypothesis of a zinc-pathway that might enhance testosterone production. Zinc may increase testosterone through two hypothetical pathways in the Leydig cells: antioxidant action and steroidogenic enzyme interaction. Intracellular increase of zinc is associated with a zinc cytoplasm increase, both at the mitochondria, nucleus and vesicle stock. (1) Zinc is absorbed by the Leydig cells mediated by ZRT1 and ZRT2 transporters. In the cytoplasm, zinc, coupled with proteins further stimulates Nrf2, such as Zinc-Protein A20, thereby neutralizing pro-oxidative and inflammatory extranuclear and intranuclear pathways which impair Leydig cell metabolism. Zinc by itself is able to directly inhibit NF-κB. (2) In the nucleus, a zinc increase through the expression of steroidogenic enzymes, such as P450c17, leads to the up-regulation of A20 mRNA. Zinc acts as a transcription factor for steroidogenic factor 1 (SF1), which is the precursor of STAR protein synthesis. (3) At the mitochondria, zinc acts with P450scc in the formation of pregnenolone through enzyme-coupled reactions. (4) In the endoplasmic reticulum, zinc may act indirectly in the final process of testosterone synthesis, since the enzyme P450c17 (which converts DHEA into testosterone) is zinc-dependent. COX-2: Ciclo-oxygenase-2; DHEA: Dehydroepiandrosterone; IL-1β: Interleukin 1; IL-2: Interleukin 2; IL-6: Interleukin 6; IL8: Interleukin 8; IL12: Interleukin 12; Nrf2: nuclear factor E2-related factor 2; NF-κB: factor nuclear kappa B; p38MAPK: p38 mitogen-activated protein kinases; P450scc: cholesterol side-chain cleavage enzyme; P450c17: steroid 17 alpha-hydroxylase/17,20 lyase; p53: Protein p53; ROS: reactive oxygen species; SF-1: Steroidogenic factor-1; STAR: steroidogenic acute regulatory protein (StAR); TNF-α: Tumor necrosis factor alpha; ZRT1: Zinc-regulated transporter 1; ZRT2: Zinc-regulated transporter 2.
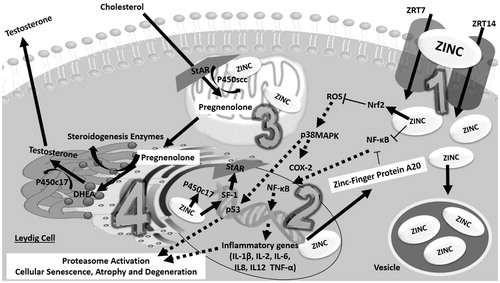
Table 1. The effect of Zinc Supplementation on parameters of the sperm analysis in infertile men.
Table 2. The effect of zinc supplementation on total testosterone in men.