Figures & data
Table 1. Baseline patient characteristics applied in the model.
Table 2. Treatment efficacy and tolerability applied in the model.
Table 3. Cost inputs applied in the model.
Table 4. Utility decrements applied in the model.
Table 5. Results (discounted) of exenatide QW and comparators: Base case and probabilistic sensitivity analysis.
Table 6. Disaggregated base case incremental cost (£) calculated for exenatide QW vs comparators.
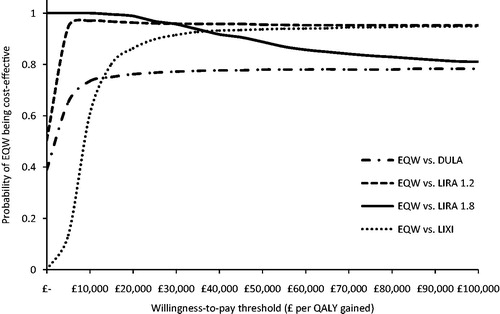
Figure 1. Cost-effectiveness acceptability curves of individual comparisons; exenatide QW vs comparators.EQW, exenatide QW; DULA, dulaglutide 1.5 mg QW; LIRA 1.2, liraglutide 1.2 mg QD; LIRA 1.8, liraglutide 1.8 mg QD; LIXI, lixisenatide 20 μg QD.