Figures & data
Figure 1. Natural history model schematic. CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SVR, sustained virologic response. Patients with HCV infection initiated treatment in the first cycle. Health states are depicted by ellipses, while arrows represent permissible transitions between health states. Hashed arrows depict the possibility of achieving SVR. Dotted arrows depict progress to HCC from any recovered states, including without history of cirrhosis and history of compensated cirrhosis. Background mortality is possible from any health state. Liver-related mortality is possible from DCC, HCC, and liver transplantation.
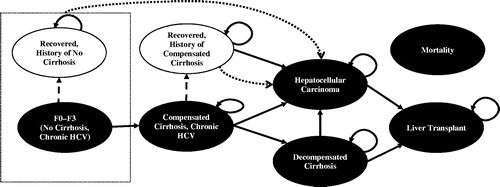
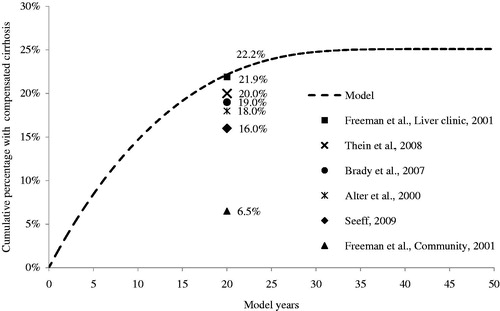
Table 1. Model inputs for the base case, deterministic, and probabilistic sensitivity analyses.
Figure 2. Liver outcomes in (a) treatment-naïve patients; and (b) treatment-experienced patients. CC, compensated cirrhosis; DCC, decompensated cirrhosis; DCV/ASV, daclatasvir/asunaprevir; HCC, hepatocellular carcinoma; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir.
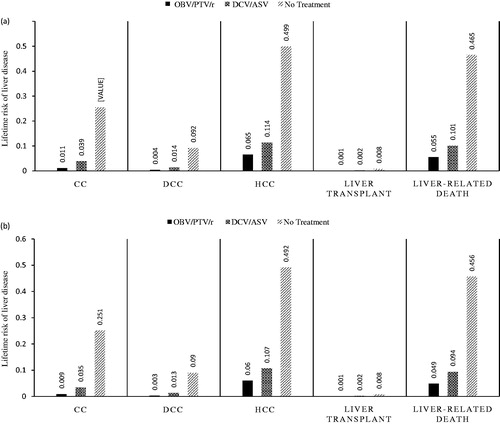
Table 2. Total costs, LYs, QALYs, ICERs, and effectiveness across patient segments.
Figure 3. OBV/PTV/r vs DCV/ASV in HCV GT1b treatment-naïve patients without cirrhosis (base case: JPY 1,684,751). CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; DCV/ASV, daclatasvir/asunaprevir; GT1b, genotype 1b; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; SVR, sustained virologic response.
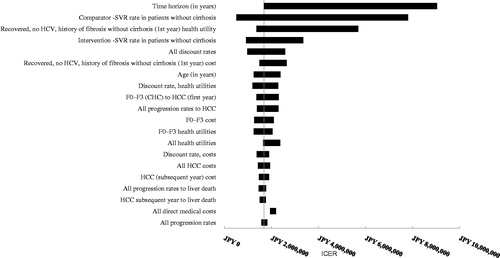
Figure 4. OBV/PTV/r vs DCV/ASV in HCV GT1b treatment-experienced patients without cirrhosis (base case: JPY 1,836,596). CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; DCV/ASV, daclatasvir/asunaprevir; GT1b, genotype 1b; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; SVR, sustained virologic response.
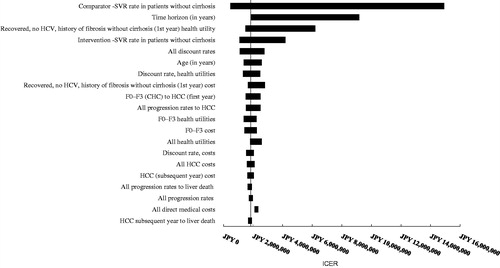
Figure 5. Probabilistic sensitivity analysis in treatment-naïve HCV GT1b patients without cirrhosis. GT1b, genotype 1b; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; WTP, willingness-to-pay. PSA estimated on 500 simulations. OBV/PTV/r is the optimal therapy in at least 86.0% of the simulations, assuming payers have a WTP threshold of JPY 5 million/QALY. The dashed line indicates the JPY 5 million WTP threshold; the data points to right of this line yield ICERs below the WTP threshold.
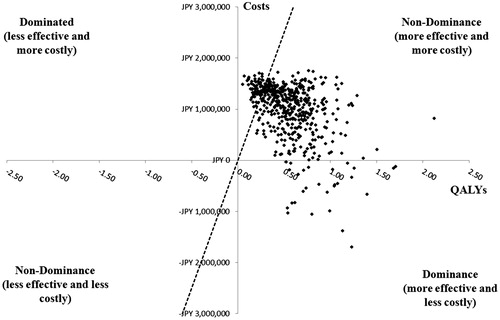
Figure 6. Probabilistic sensitivity analysis in treatment-experienced patients with HCV GT1b infection without cirrhosis. GT1b, genotype 1b; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; WTP, willingness-to-pay. PSA estimated on 500 simulations. OBV/PTV/r is the optimal therapy in at least 88.2% of the simulations, assuming payers have a WTP threshold of JPY 5 million/QALY. The dashed line indicates the JPY 5 million WTP threshold; the data points to right of this line yield ICERs below the WTP threshold.
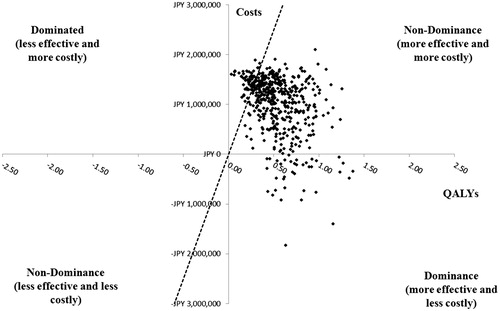
Table 3. Total costs, QALYs, and ICER results for patients without the Y93H mutation.
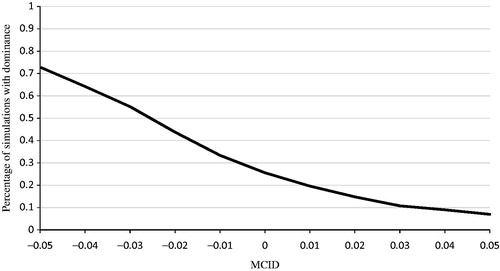
Figure 7. Probabilistic sensitivity analysis: results using MCID of ±0.05 in QALYs between OBV/PTV/r and SOF/LDV. MCID, minimally clinical important difference; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; SOF/LDV, sofosbuvir/ledipasvir. PSA estimated on 500 simulations. OBV/PTV/r is a dominant strategy (i.e. less costly and more effective) vs SOF/LDV in 25.6% of simulations (i.e. MCID ≥0). In addition, when we varied the MCID in QALYs from 0.05 to –0.05, OBV/PTV/r dominates SOF/LDV in 7.0–73.0% of simulations.