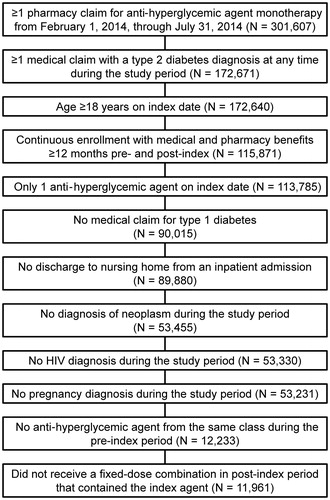
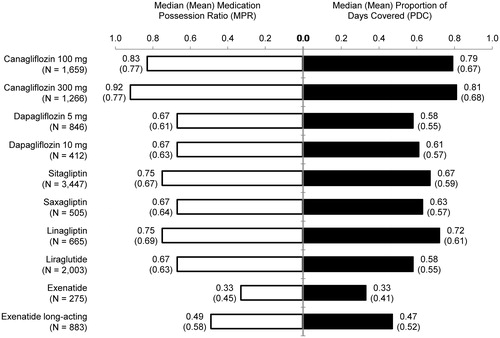
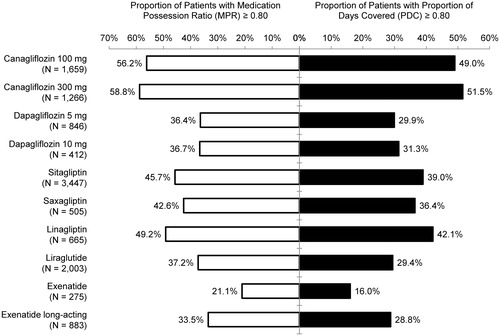
Figures & data
Table 1. Key baseline characteristics, index costs, and days of supply by index anti-hyperglycemic agent.
Figure 2. Kaplan-Meier analysis of persistence, using the time to treatment discontinuation. Cana, canagliflozin; Dapa, dapagliflozin; Exen, exenatide; Exen LA, long-acting exenatide; Lina, linagliptin; Lira, liraglutide; Saxa, saxagliptin; Sita, sitagliptin.
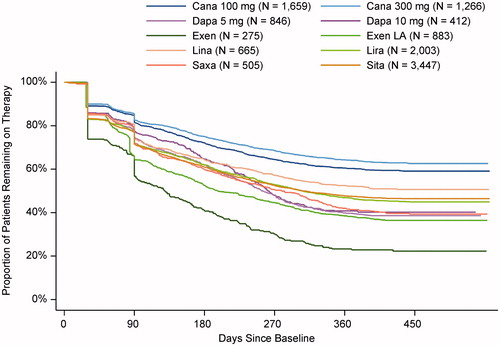
Figure 3. Cox proportional hazards model ratios for treatment non-persistence. * p < 0.05 vs reference. † Included combinations with the previous items in the list and excluded combinations with the subsequent items. For example, “DPP-4 inhibitors” included DPP-4 inhibitors either alone or in combination with metformin, sulfonylureas, meglitinides, α-glucosidase inhibitors, and/or thiazolidinediones; and excluded combinations of DPP-4 inhibitors with GLP-1 agonists, SGLT2 inhibitors, or insulin. Co-payment/co-insurance for each pharmacy claim was adjusted to 30 days. CDHP, consumer-directed health plan; CI, confidence interval; DCSI, Diabetes Complications Severity Index; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors (e.g. sitagliptin, saxagliptin, linagliptin); EPO, exclusive provider organization; GLP-1 agonists, glucagon-like peptide-1 receptor agonist (e.g. exenatide, liraglutide); HDHP, high-deductible health plan; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization; SGLT2 inhibitors, sodium/glucose cotransporter 2 inhibitors (e.g. canagliflozin, dapagliflozin).
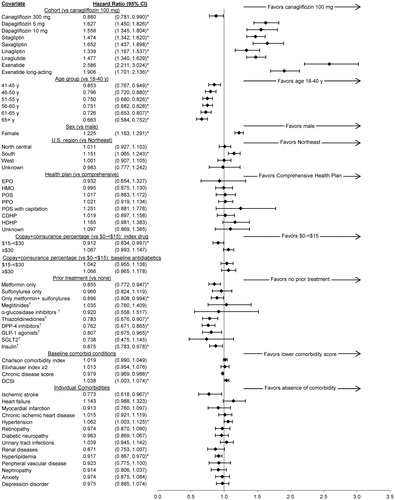
Table 2. Odds ratios for treatment adherence, defined as either proportion of days covered ≥80% or medication possession ratio ≥80%, using multivariable logistic regression analyses.