Figures & data
Figure 1. Model structure, Adapted from Gani et al.Citation20. Ovals represent health states. All health states may progress to death. Rectangles represent events that patients can experience at any time. Treatment-related AEs and treatment discontinuation can only occur for patients receiving treatment. AE, adverse event; EDSS, Expanded Disability Status Scale; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary-progressive multiple sclerosis; State N = current EDSS state.
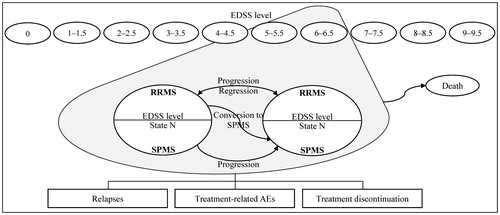
Table 1. Initial EDSS distribution of the cohort, ARR natural history, utility, costs of disease management, and mortality by EDSS level.
Table 2. Comparative efficacy, discontinuation risk, and AE-associated utility decrement.
Table 3. Treatment-related costs.
Figure 2. ARR validation: Model outcomes vs ADVANCE trial after 1 year. ARR, annualized relapse rate.
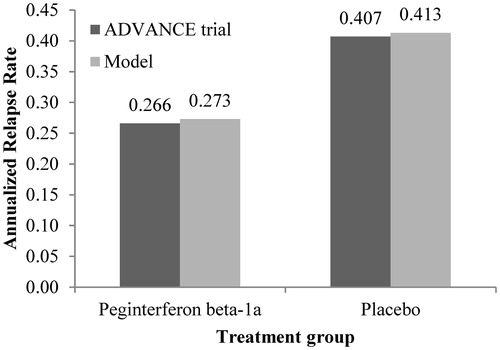
Figure 3. EDSS distribution validation: Model outcomes vs ADVANCE trial after 1 year—Peginterferon beta-1a arm. EDSS, Expanded Disability Status Scale.
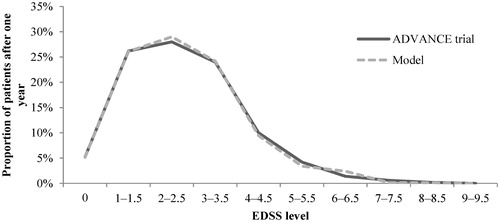
Figure 4. EDSS distribution validation: Model outcomes vs ADVANCE trial after 1 year—Peginterferon beta-1a arm. EDSS, Expanded Disability Status Scale.
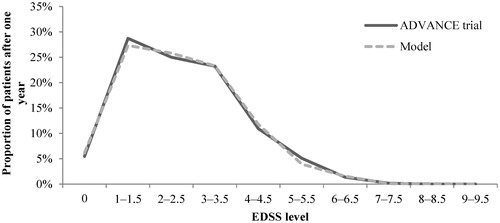
Table 4. Base case outcomes over 30 years.
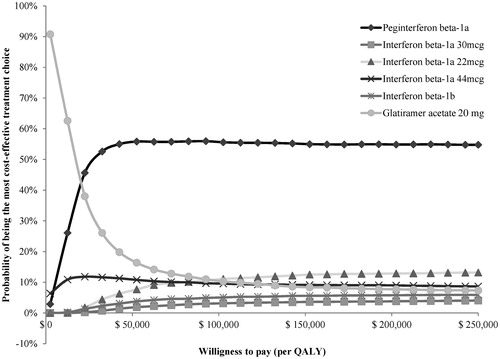
Figure 5. Multi-way cost-effectiveness acceptability curves over 30 years. mcg, microgram; QALY, quality-adjusted life-year.