Figures & data
Figure 1. Semi-Markov model structure. BSC: best supportive care; mCRC: metastatic colorectal cancer. 1a: Progression Free to Death; 1b: Progression Free to Progressive Disease: Treat with Subsequent Active Therapy; 1c: Progression Free to Progressive Disease: Treat with Best Supportive Care; 1d: Progression Free to Attempted Resection of Metastases; 2a: Progressive Disease: Treat with Subsequent Active Therapy to Death; 2b: Progressive Disease: Treat with Subsequent Active Therapy to Progressive Disease: Treat with Best Supportive Care; 3: Progressive Disease: Treat with Best Supportive Care to Death; 4a: Attempted Resection of Metastases to Progression Free; 4b: Attempted Resection of Metastases to Death; 4c: Attempted Resection of Metastases to Disease Free After Metastases Resection; 5a: Disease Free After Metastases Resection to Death; 5b: Disease Free After Metastases Resection to Progressive Disease: After Resection and Relapse; 6: Progressive Disease: After Resection and Relapse to Death.
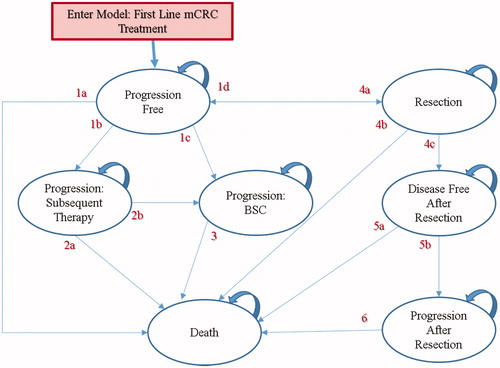
Figure 2. Survival curves. (a) Progression-free survival; (b) Overall survival. Bmab: bevacizumab; Pmab: panitumumab.
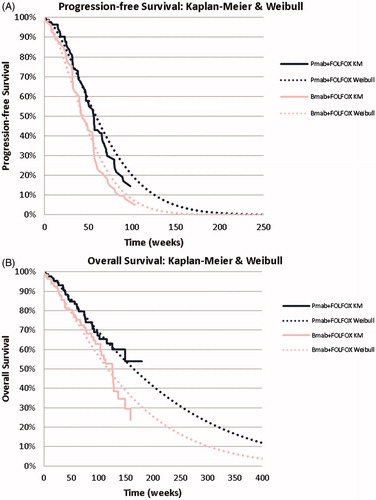
Table 1. Drug acquisition and administration costs.
Table 2. Incidence/costs of serious adverse events and other medical costs.
Table 3. Utility weight values.
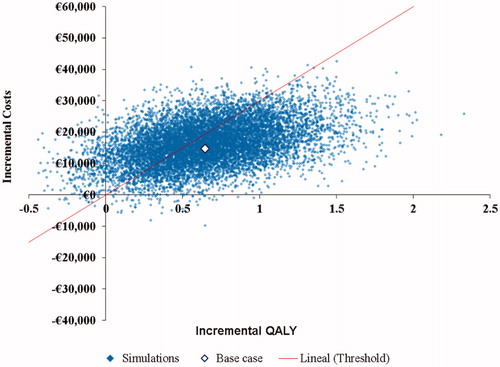
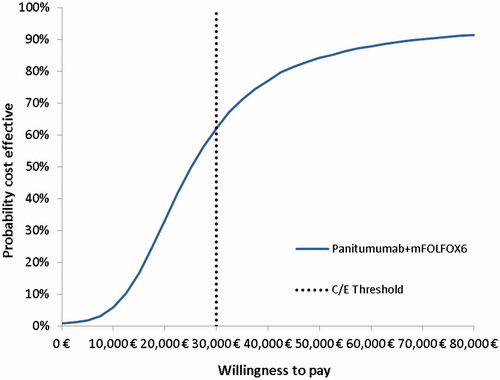
Table 4. Base case results of the cost-effectiveness analysis.
Table 5. Scenario analyses results.