Figures & data
Table 1. Baseline characteristics of modeled populations, based on ITT in EINSTEIN phase III clinical study program (combined across treatment arms).
Figure 1. Model structure depicting both the index-PE and index-DVT module. Dashed health states/arrows indicate additional states/pathways in index-PE module with respect to index-DVT module. All patients who have had a PE are at risk of CTEPH, with the exception of those in the acute IC and post-IC bleed states (as these states are considered worse than CTEPH). On Tx, On treatment; Off Tx, Off treatment; rVTE, Recurrent VTE event; DVT, Deep-vein thrombosis (ipsilateral and contralateral); PE, Pulmonary embolism; PTS, Post-thrombotic syndrome; CTEPH, Chronic thromboembolic pulmonary hypertension; IC, Intracranial; EC, Extracranial; CRNM, Clinically relevant non-major. *Health states with increased mortality risk. ‡ Health state in the index-PE module for patients who have experienced a DVT and are, therefore, at risk of PTS. † PTS costs and utilities are applied to proportion of patients in the Off Tx post-DVT state.
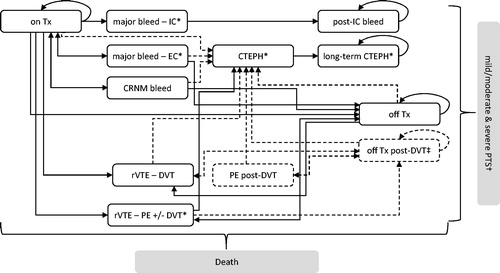
Table 2. Baseline risks (LMWH/VKA) and hazard ratios (rivaroxaban) based on the pooled EINSTEIN trial data (DVT/PE). Figures regarding major bleed are based on the first major bleed event (Prins et al.Citation25 considered first bleed event).
Table 3. Health state utility weights, as applied in the cost-effectiveness analysis.
Table 4. Quarterly treatment-related costs (2015).
Table 5. Quarterly event-associated costs (2015).
Table 6. Event and treatment-related number of days absent from work.
Table 7. Lifetime time-horizon results (base case: costs discounted at 4% and effects at 1.5%).
Figure 2. Tornado diagram for total discounted (1.5%) incremental QALYs per patient (top 15 parameters).
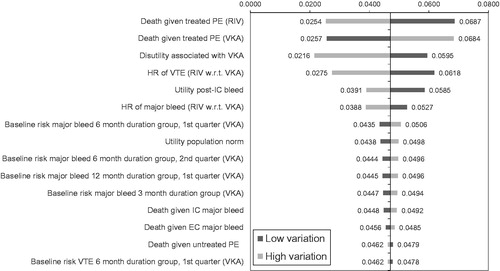
Figure 3. Tornado diagram for total discounted (4%) incremental costs per patient (top 15 parameters).
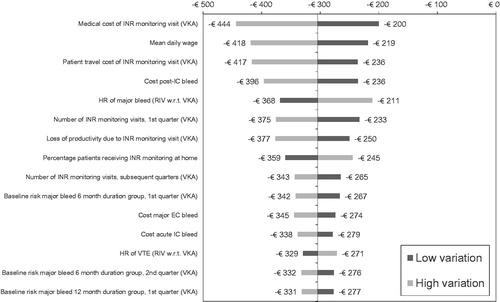
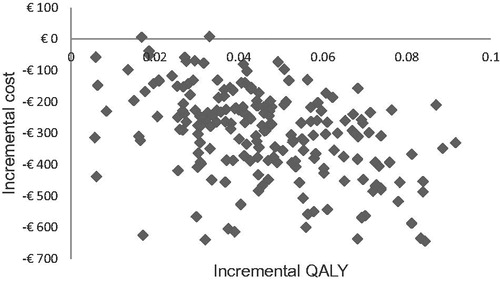
Figure 4. Scatter plot of probabilistic sensitivity analysis; incremental costs per patient vs incremental QALYs per patient.