Figures & data
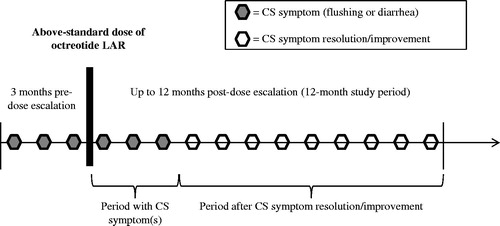
Table 1. Clinical inputs from the NET 3-center chart review study for the cost model.
Table 2. Cost inputs from claims data analysis.
Table 3. Clinical inputs for patients under 65 years of age from the Net 3-Center chart review study.
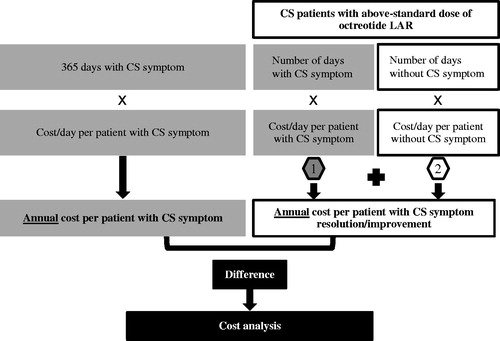
Table 4. Cost reduction associated with CS symptom resolution or improvement: main analysis, healthcare cost.
Table 5. Cost reduction associated with CS symptom resolution or improvement: sensitivity analysis, direct healthcare, and indirect work loss costs.