Figures & data
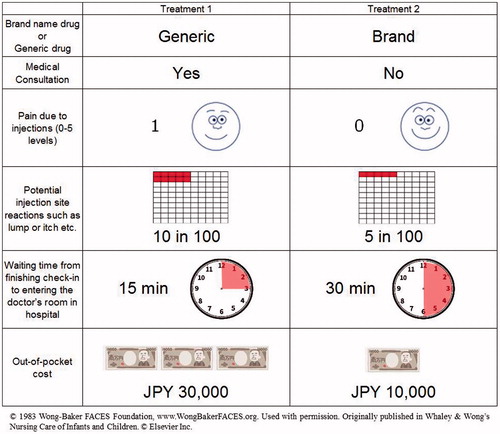
Table 1. Attributes and attribute levels in discrete choice experiment.
Table 2. Medical cost reduction for prostate cancer patients with injections of leuprorelin acetate.
Table 3. Intangible costs for one injection by discrete choice experiment.
Table 4. Intangible costs saved for having one less injection in prostate cancer patients with injections of leuprorelin acetate. Note that intangible costs saved for having one less injection were calculated by the results of discrete choice experiment on respondents without prostate cancer.
Table 5. Sensitivity analysis of intangible costs saved for having one less injection in prostate cancer patients with injections of leuprorelin acetate.
Table 6. Total costs reduction for having one less injection in prostate cancer patients with injections of leuprorelin acetate.