Figures & data
Figure 1. Schematic representation of the cost-effectiveness model structure. Abbreviations. Dmab, denosumab; MM, multiple myeloma; Tx, treatment; ZA, zoledronic acid.
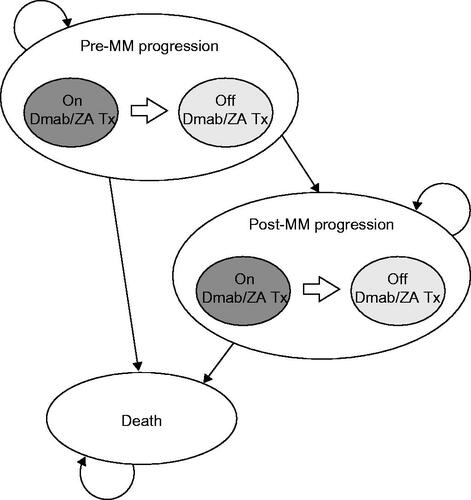
Table 1. Model economic inputs by country.
Table 2. Base case cost-effectiveness analysis (discounted) by country.
Table 3. Alternative scenario cost-effectiveness analyses (discounted) by country.
Figure 2. Main drivers of uncertainty in cost-effectiveness analysis by country. The ICER is the cost (€) per QALY gained derived from univariate analyses employing the lower and upper bounds of top five key model inputs. Cross-hatching represents situations in which Dmab is dominant vs ZA (Dmab is associated with more QALYs and fewer overall costs than ZA). The ranges for the model parameters are provided in Supplementary Table S4. Abbreviations. Dmab, denosumab; ICER, incremental cost-effectiveness ratio; i.v., intravenous; MM, multiple myeloma; QALY, quality-adjusted life-year; SRE, skeletal-related event; ZA, zoledronic acid.
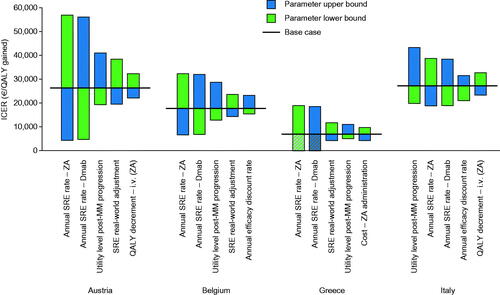
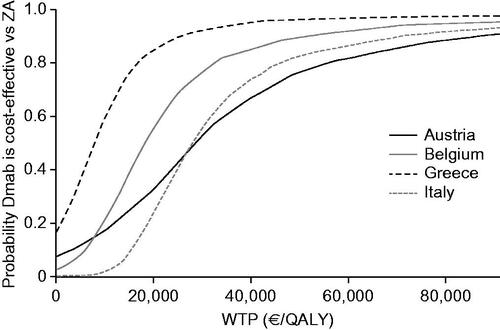
Figure 3. Cost-effectiveness acceptability curves by country. The model input parameters are provided in Supplementary Table S5. Abbreviations. Dmab, denosumab; QALY, quality-adjusted life-year; WTP, willingness to pay; ZA, zoledronic acid.