Figures & data
Figure 1. Schematic visualization of the decision-tree model. Over each branch, a brief description of the corresponding patient pathway has been summarized. Abbreviations. CAP, community-acquired pneumonia; ICU, intensive care unit; IV, intravenous.
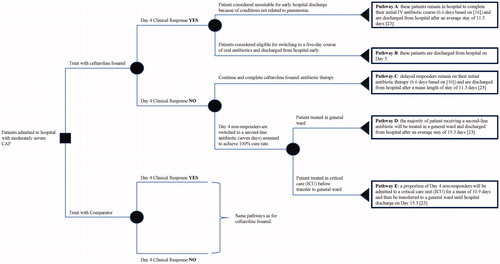
Table 1. Clinical inputs for the cost-effectiveness analysis.
Table 2. Economic inputs for the cost-consequences analysis.
Figure 2. Comparison of total costs per patient (left side) and the percentage of patients discharged early (right side) for CFT, Ceftriaxone, and Levofloxacin. Abbreviations. CFT, ceftaroline fosamil; q12hr, every 12 hours; q24hr, every 24 hours
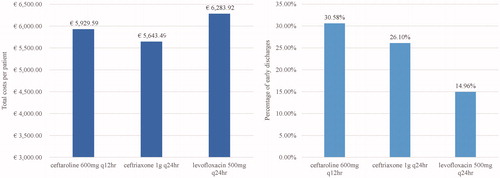
Table 3. Total and incremental percentage of patients, for each treatment pathway (according to ), predicted by the model.
Table 4. Total and incremental hospital costs per patient by antibiotic regimen and pneumonia type.
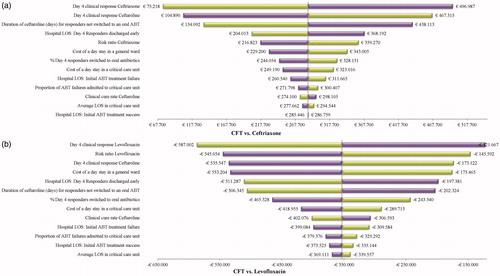
Figure 3. Deterministic sensitivity analysis results: CFT 600 mg q12hr versus Ceftriaxone 1 g q24hr (a); CFT 600 mg q12hr versus Levofloxacin 500 mg q24hr (b). Abbreviations. ABT, antibiotic therapy; CFT, ceftaroline fosamil; LOS, length of stay; q12hr, every 12 hours; q24hr, every 24 hours.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions see (https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
