Figures & data
Figure 1. Partitioned survival model health state transitions. Abbreviations: PF: progression-free; PD: progressed disease.
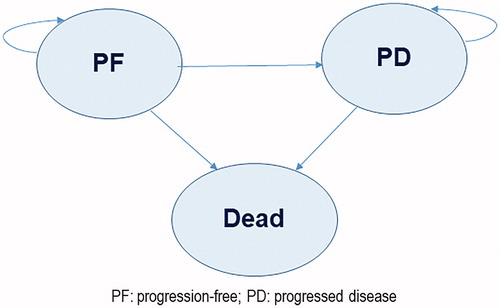
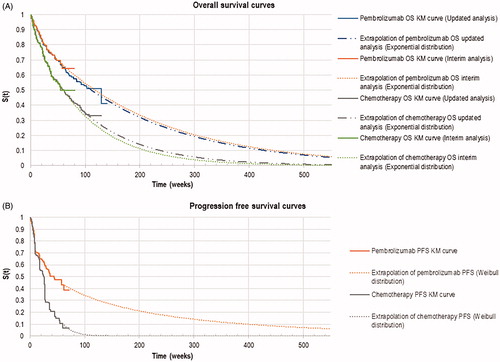
Table 1. Key input data for base-case analysis.
Table 2. Results of base-case and scenario analyses.
Figure 3. One-way sensitivity analysis tornado diagram for pembrolizumab versus chemotherapy (base-case ICER: SGD167,692 per QALY). Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, Quality-adjusted life-years; SGD, Singapore dollars; 1L, first-line; 2L, second line; AE, adverse events.
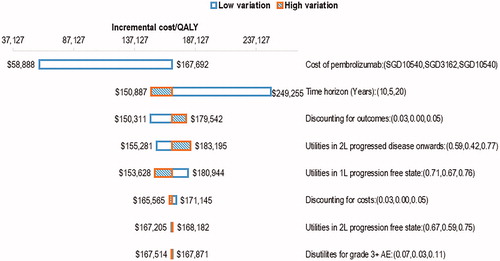
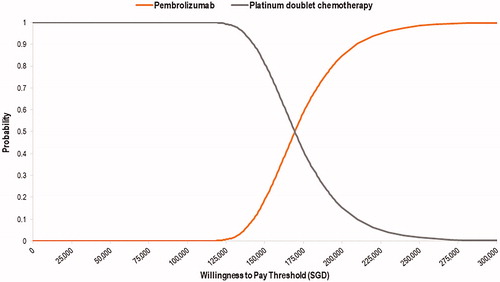
Figure 4. Cost-effectiveness acceptability curve showing the likelihood of pembrolizumab being cost-effective compared to platinum-based chemotherapy across different willingness-to-pay thresholds.