Figures & data
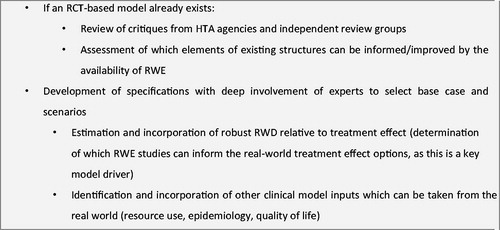
Table 1. RWE used as a key source of inputs.
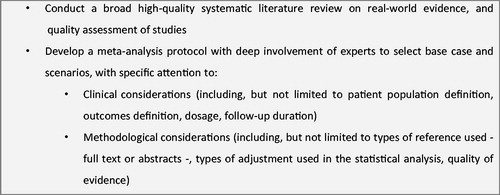
Table 2. Items related to relative treatment effect in existing tools to evaluate cost-effectiveness analyses.