Figures & data
Figure 1. Patient disposition. AF, atrial fibrillation; VTE, venous thromboembolism. Note. 1. Patients initiated on treatment with both rivaroxaban and apixaban on the index date were classified as apixaban, and subsequently excluded (n = 16).
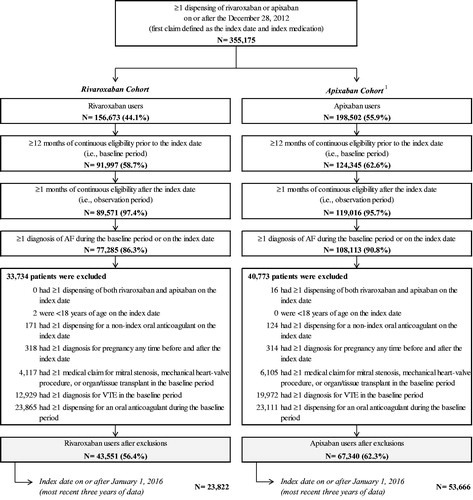
Table 1. Baseline demographics and clinical characteristics – unweighted and weighted rivaroxaban and apixaban cohorts.
Table 2. NVAF-related comorbidities and risk factors for stroke/systemic embolism and major bleeding – unweighted and weighted rivaroxaban and apixaban cohorts.
Table 3. All-cause healthcare resource utilization of the rivaroxaban and apixaban cohorts – up to 18 and 24 months follow-up.
Table 4. Healthcare cost components of the rivaroxaban and apixaban cohorts – up to 18 and 24 months follow-up.
