Figures & data
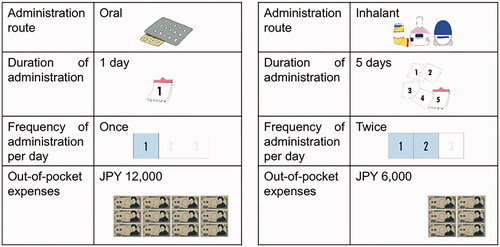
Table 1. Attributes and attribute levels in the survey for the conjoint analysis.
Table 2. Number of respondents by age and gender.
Table 3. Number of respondents by dosage forms after cross-tabulation based on answers to questions (Q5) “Which type of antiviral agent did you take the last time?” and (Q6) “What kind of dosage did you take of (or how to use) the antiviral agent?”
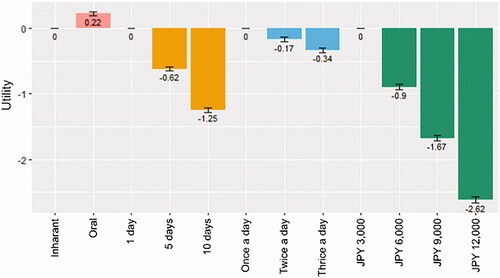
Table 4. Monetary value of each level of each attribute estimated by the baseline model.
Figure 3. Difficulty in taking the drug (answer to Q 8-1: “Was it easy to take the antiviral that you took the last time?”) (A), and reasons for the difficulty (answer to Q 8-2: “Why did you answer ‘Neither’ or ‘No’ to Q8-1 regarding the ease of taking the antiviral that you took the last time?”) by type of drug (B). Multiple choices were allowed. Denominator: All respondents according to the type of dosage form of the drugs.
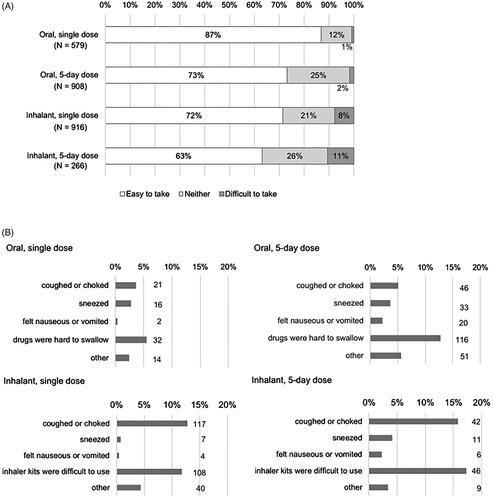
Figure 4. Feeling of worry about taking the drug (answer to Q 9: “When you were taking the antiviral for influenza the last time, were you worried that you would vomit out the drugs or you did not take (inhale) enough of the drugs?”) by type of dosage form of the drugs (A) or by age and gender (B).
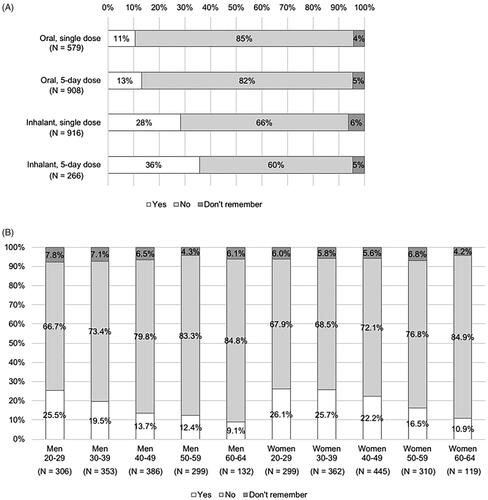
Figure 5. Number of days to fever resolution from initiation of the antivirals (answer to Q 10: “When you were taking the antiviral for influenza the last time, how long did it take to break your fever after the first dose?”) by dosage form of the drugs identifying the percentages of respondents excluding those who answered “not sure” for each answer. N: number of respondents excluding “not sure” (oral, single dose: 55; oral, 5-day dose: 82; inhalant, single dose: 98, and inhalant, 5-day dose: 19).
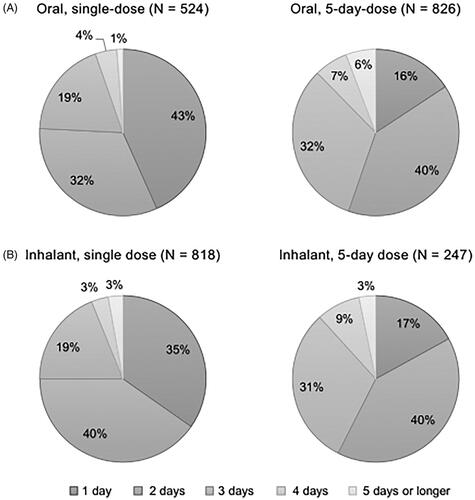
Figure 6. Number of days to return to the usual life from initiation of the antivirals (answer to Q 11: “When you were taking the antiviral for influenza the last time, how long did it take to return to your usual life (working or attending school) after the first dose?”) by type of dosage form of the drugs identifying the percentages of respondents excluding those who answered “not sure” for each answer. N: number of respondents excluding “not sure” (oral, single dose: 47; oral, 5-day dose: 60; inhalant, single dose: 86, and inhalant, 5-day dose: 12).
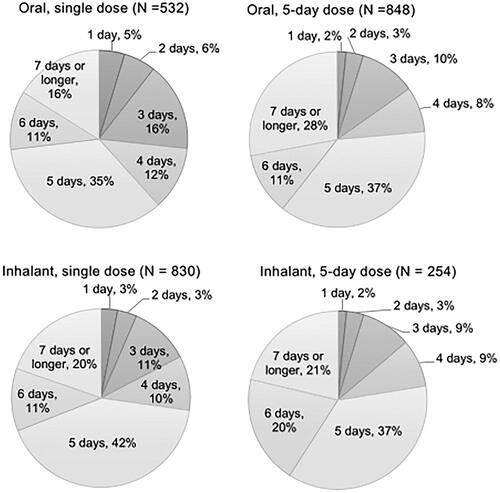
Table 5. Estimated total cost of the drug, utility, and productivity loss for influenza per patient for available antivirals in Japan.