Figures & data
Figure 1. Age distribution at index date by (a) inclusion criteria cohort in patient identification analysis and (b) time period in temporal trend analysis. Abbreviations. NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; Sx, symptom; TM, transverse myelitis.
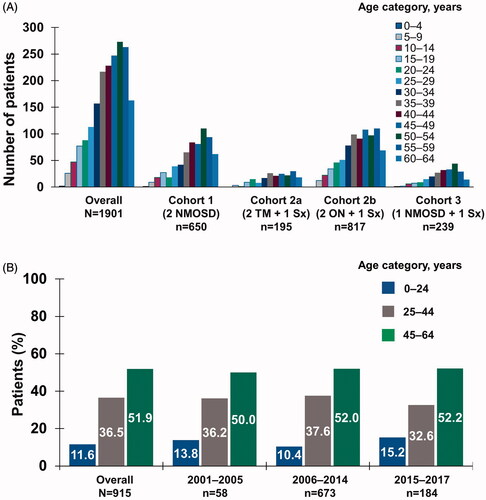
Figure 2. Use of diagnostic testing by (a) inclusion criteria cohort in patient identification analysis and (b) time period in temporal trend analysis. Abbreviations. AQP4-IgG, aquaporin-4–immunoglobulin G; MRI, magnetic resonance imaging; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; Sx, symptom; TM, transverse myelitis.
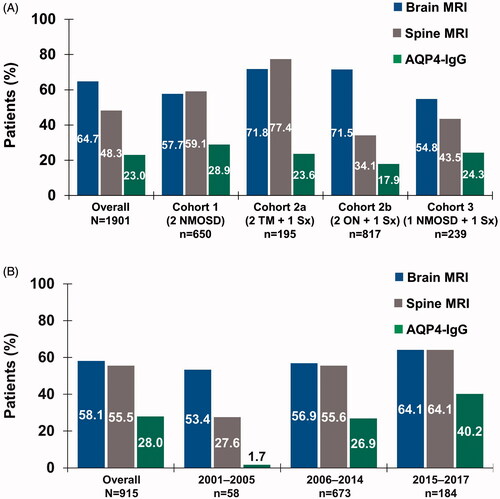
Figure 3. (a) IST and (b) disease-modifying therapy use by inclusion criteria cohort, before index date and after NMOSD diagnosis in patient identification analysis. (c) IST and (d) disease-modifying therapy use by time period in temporal trend analysis. Abbreviations. IST, immunosuppressive therapy; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; Sx, symptom; TM, transverse myelitis.
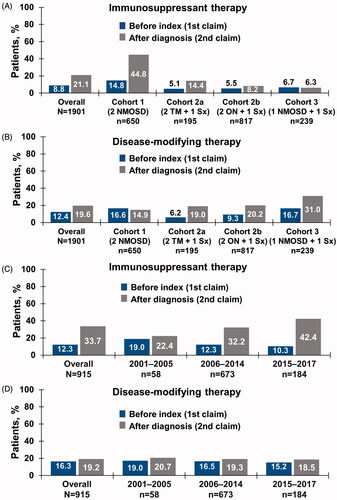
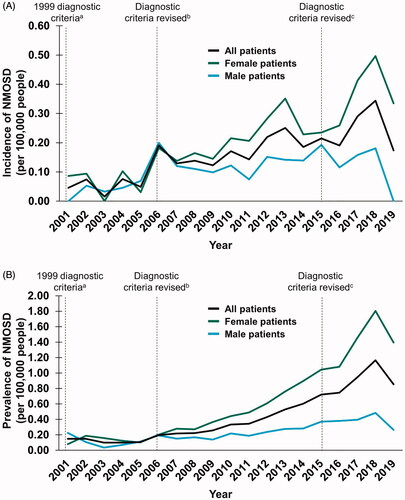
Figure 4. Temporal trends in the (a) incidence and (b) prevalence of NMOSD between 2001 and 2019. a Criteria stipulated ON, acute myelitis and no symptoms indicative of CNS involvementCitation19; b Consensus criteria were revised to include requirements for ≥ 2 of the following: MRI evidence of a contiguous spinal cord lesion ≥ 3 segments in length, onset brain MRI nondiagnostic for MS or NMO-IgG seropositivityCitation20; c Consensus criteria were revised to include both NMOSD with AQP4-IgG and NMOSD without AQP4-IgG. An additional category of NMOSD with unknown AQP4-IgG status may be used for patients in whom serologic testing is unavailableCitation11. Abbreviations. AQP4-IgG, aquaporin-4–immunoglobulin G; MRI, magnetic resonance imaging; MS, multiple sclerosis; NMO, neuromyelitis optica; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; TM, transverse myelitis.
Supplemental Material
Download MS Word (135.5 KB)Data availability statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
