Figures & data
Table 1. Reported test-specific adherence rates.
Table 2. Discounted clinical outcomes per 1,000 patients.
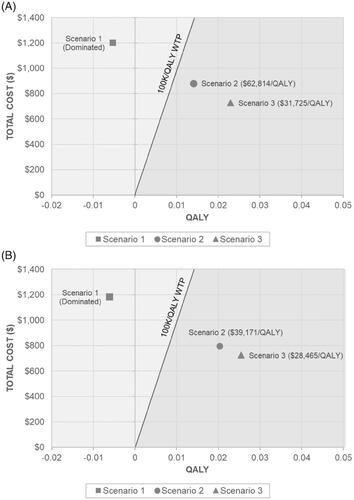
Table 3. Discounted total costs and QALYs with associated incremental cost-effectiveness ratios.
Table 4. ICER for capped adherence sensitivity analyses.
Figure 2. One-way sensitivity analysis of incremental cost-effectiveness of mt-sDNA vs. (A) FIT or (B) FOBT (scenario 3).
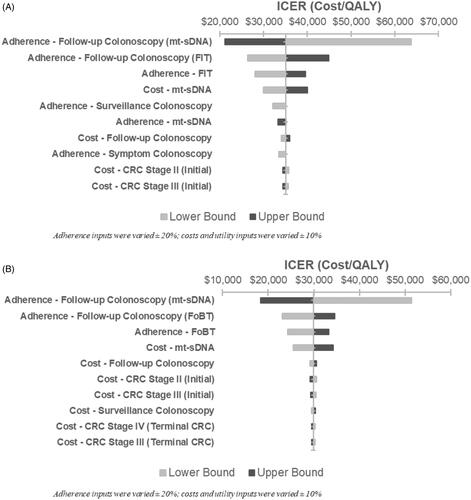
Figure 3. Heatmap of mt-sDNA vs. FIT (A) and mt-sDNA vs. FOBT (B) when varying screening test adherence (follow-up colonoscopy adherence fixed at 100%).
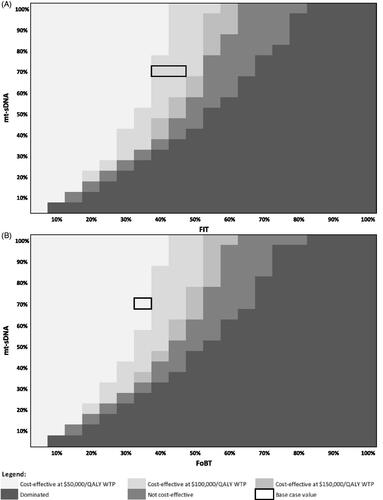
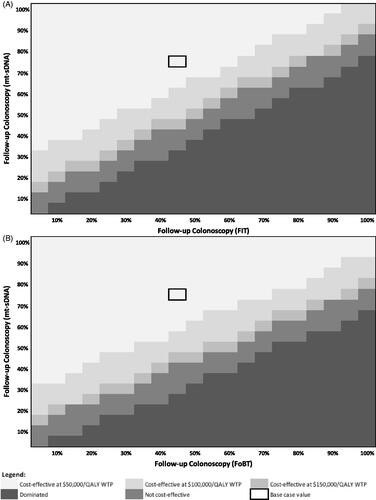
Figure 4. Heatmap of mt-sDNA vs. FIT (A) and mt-sDNA vs. FOBT (B) when varying follow-up colonoscopy adherence (screening test rates fixed).