Figures & data
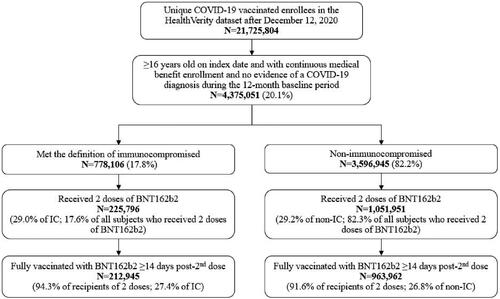
Figure 1. Algorithm to define IC cases. aIndex date was defined as the date of receipt of the 1st BNT162b2 dose; bbased on a review of ICD-10 diagnosis codes and keyword search including immune, malignancy, neoplasm, rheumatoid, and kidney, excluding encounter/screening test. Abbreviations. AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; IC, immunocompromised; IS, immunosuppressive.
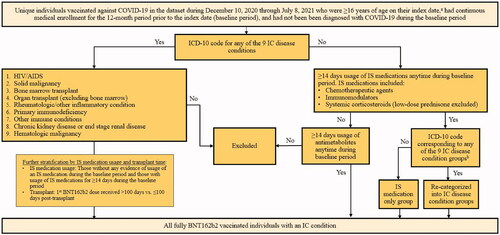
Table 1. Characteristics of the IC and non-IC cohorts and overall study population that received 2 BNT162b2 doses.
Table 2. Incidence rates of COVID-19 vaccine breakthrough infections per 100 person-years among individuals fully vaccinateda with BNT162b2, by IC condition and age <65 and ≥65 years.
Table 3. Characteristics of fully vaccinateda individuals with COVID-19 vaccine breakthrough infections.
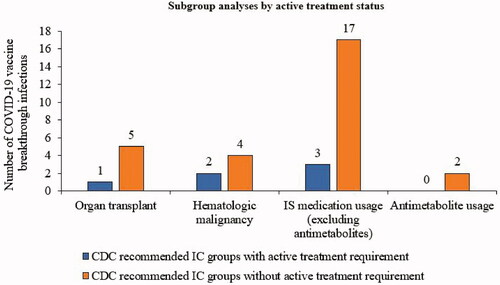
Figure 3. COVID-19 vaccine breakthrough infections: subgroup analysis of CDC recommended IC groups by active treatment status. Active treatment was defined as evidence of medication usage ≤14 days before a 1st BNT162b2 dose, except for hematological malignancy, where active treatment status was defined as evidence of any IS medication (including radiotherapy) usage within 6 months before a 1st BNT162b2 dose. Abbreviations. CDC, Centers for Disease Control and Prevention; IC, immunocompromised; IS, immunosuppressive.
Supplemental Material
Download MS Word (44.5 KB)Data availability statement
Data generated or analyzed during this study are available upon request.