Figures & data
Figure 1. Study flow of Canadian hospital abstracts. †ICD-10-CA diagnosis code listed as type “most responsible diagnosis”, “pre-admit comorbidity” or “post-admit comorbidity” (DAD); or as type “main problem” or “other problem” (NACRS). Abbreviations. CSHS, cost of a standard hospital stay; DAD, Discharge Abstract Database; ED, emergency department; ICD-10-CA, ICD-10-CA, International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada; n, number of hospital abstracts; NACRS, National Ambulatory Care Reporting System; RIW, resource intensity weight.
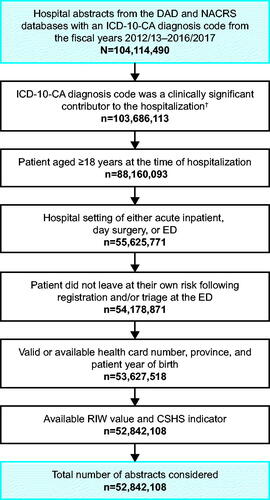
Table 1. Annual hospitalization costs with degludec and glargine U100.
Table 2. Aggregate hospitalization costs by SOC.
Table 3. Top 10 single preferred term drivers and annual hospitalization costs with degludec and glargine U100.
Supplemental Material: Appendix A
Download MS Word (421.5 KB)Supplemental Material: Appendix B
Download MS Excel (144.5 KB)Data availability statement
All estimates for unit hospitalization costs are available in the appendices, while the SAE data from DEVOTE are publicly available at ClinicalTrials.gov or in the DEVOTE primary publication. The DEVOTE clinical trial report is available from the corresponding author on request. The raw CIHI data include some personal health information and will not be made publicly available.
