Figures & data
Figure 1. Trilaciclib transiently arrests the cell cycle of HSPCs in the presence of chemotherapy to reduce the incidence of chemotherapy-induced myelosuppression. Abbreviations. CDK4/6, cyclin-dependent kinase 4 and 6; HSPC, hematopoietic stem and progenitor cell.
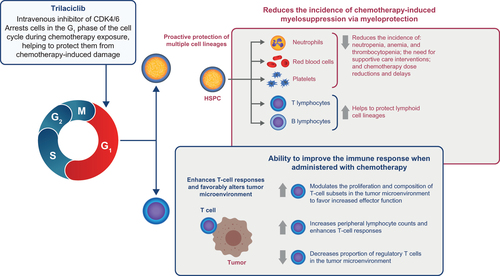
Table 1. Main parameters of the economic evaluation.
Table 2. Model parameters and assumptions.
Figure 2. Study schematic to estimate the value of trilaciclib for patients with ES-SCLC receiving first-line chemotherapy. Abbreviations. AE, adverse event; CEA, cost-effectiveness analysis; E/P/A, etoposide, carboplatin and atezolizumab; ES-SCLC, extensive-stage small cell lung cancer; QALY, quality-adjusted life year.
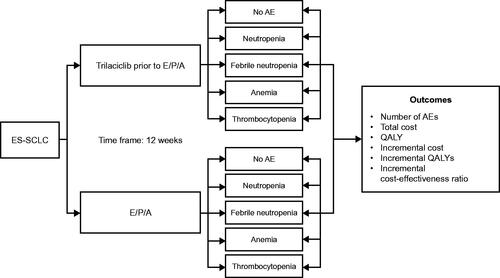
Table 3. Deterministic (base-case) results for first-line treatment of patients with ES-SCLC.
Figure 3. Incremental costs estimated from OWSAs. aUnderlying AE event rate and episode frequency were applied to both trilaciclib prior to E/P/A and E/P/A alone. bRRR of trilaciclib prior to E/P/A vs E/P/A alone. Abbreviations. AE, adverse event; E/P/A, etoposide, carboplatin, and atezolizumab; OWSA, one-way sensitivity analysis; RRR, relative risk reduction; USD, United States dollars.
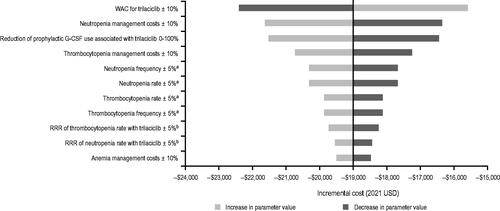
Figure 4. Cost-effectiveness plane (A) and cost-effectiveness acceptability curve (B) for the PSA. Abbreviations. E/P/A, etoposide, carboplatin, and atezolizumab; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; USD, United States dollars; WTP, willingness-to-pay threshold.
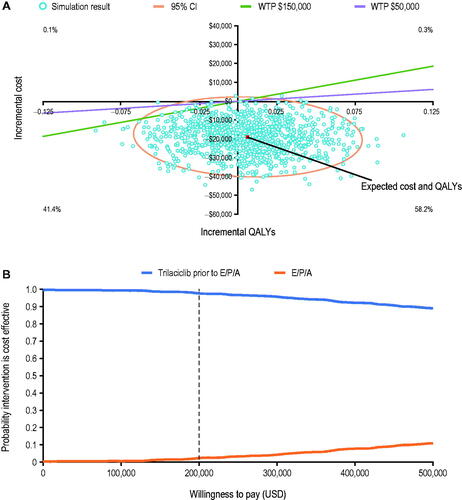
Table 4. PSA results.
Supplemental Material: Interview with Authors
Download MS Word (26.6 KB)Supplemental Material
Download MS Word (24.1 KB)Data availability statement
The data that support the findings of this study are available from the co-author, H.H., upon reasonable request.
