Figures & data
Figure 1. Flow diagram. Abbreviations. DB, double-blind, placebo-controlled trial; LUR-LUR, lurasidone-lurasidone; N, sample size; OLE, open-label extension; PBO-LUR, placebo-lurasidone.
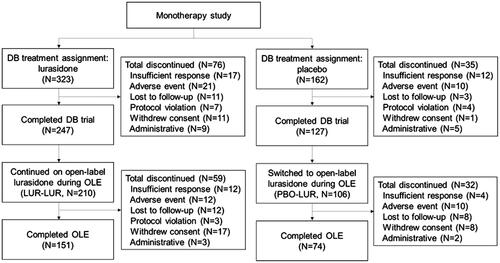
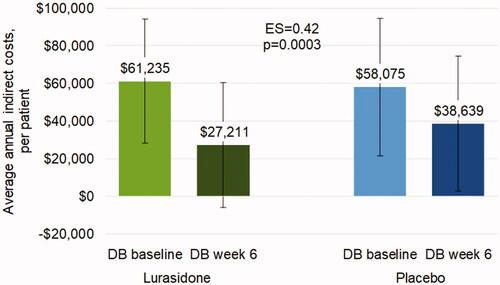
Table 1. Patient demographics and clinical characteristics at 6-week double-blind trial baseline.
Table 2. Mean changes in SDS total and item scores during 6-week double-blind trial.
Table 3. Mean changes in SDS total and item scores during 6-week double-blind trial and 6-month open-label extension in patients who continued or switched to open-label lurasidone monotherapy.
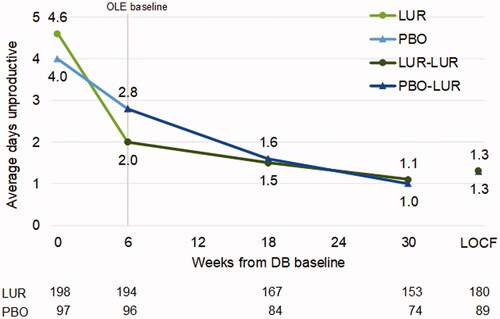
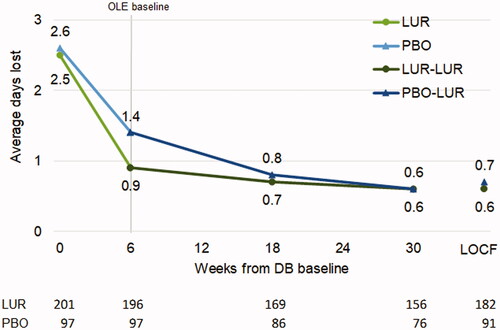
Figure 2. Average days lost in patients who continued or switched to lurasidone monotherapy for OLE. Abbreviations. DB, double-blind, placebo-controlled trial; LOCF, last observation carried forward; LUR, lurasidone; OLE, open-label extension; PBO, placebo.
Supplemental Material
Download MS Word (87 KB)Data availability statement
This post-hoc analysis used confidential clinical trial data, which are not publicly available.