Figures & data
Figure 1. Model structure: (a) phase 1 – acute care from hospital admission to 30 days; (b) phase 2 – long-term care from 30 days to model time horizon. Abbreviations. ICH, intracranial hemorrhage; mech. vent., mechanical ventilation; mRS, modified Rankin score; PCC, prothrombin complex concentrate.
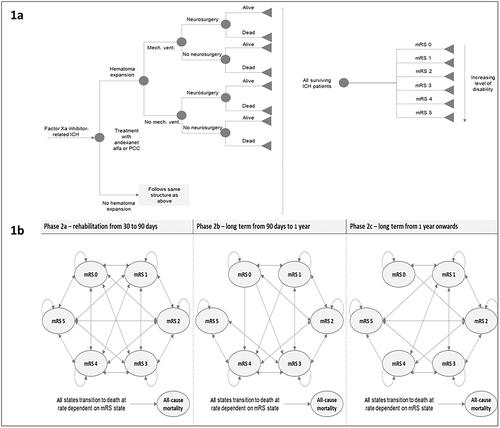
Table 1. Treatment-dependent and treatment-independent clinical inputs.
Table 2. Treatment-dependent and treatment-independent cost data.
Table 3. Base-case ICERs: payer perspective (in 2020 USD).
Figure 2. Results from deterministic sensitivity analysis for cost-effectiveness analysis. Abbreviations. mRS, modified Rankin score; PCC, prothrombin complex concentrate; QALY, quality-adjusted life-year; WAC, wholesale acquisition cost.
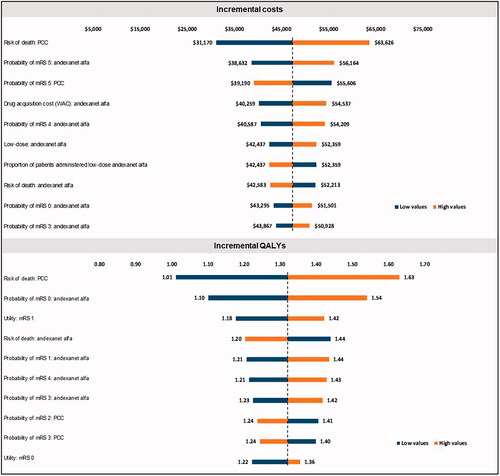
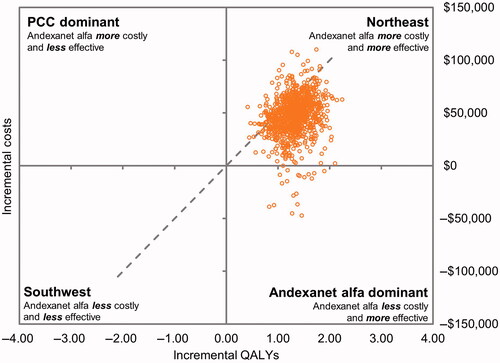
Figure 3. Results from probabilistic sensitivity analysis for cost-effectiveness analysis. Abbreviations. PCC, prothrombin complex concentrate; QALY, quality-adjusted life-year.
Supplemental Material
Download MS Word (79.9 KB)Data availability statement
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
