Figures & data
Figure 1. Schematic diagram of the decision tree model used for traditional carrier screen workflow (A) and reflex sgNIPT carrier screen workflow (B).
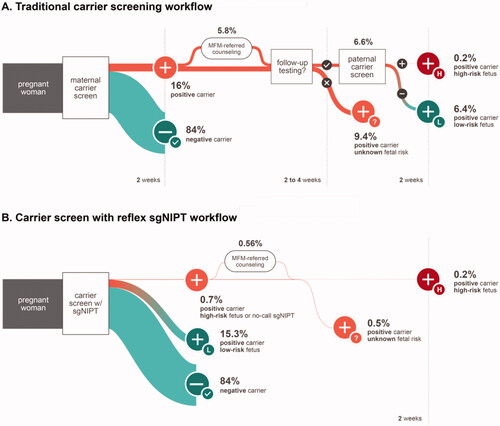
Table 1. A summary of clinical decision probabilities and test performance as part of the key assumptions for the decision-analytic model used in our analyses.
Table 2. Clinical outcomes per 100,000 individuals screened in the base and reflex sgNIPT scenarios of our decision-analytical model and the differences between these two scenarios.
Table 3. Total cost savings from reflex sgNIPT follow-up testing costs per 100,000 pregnancies.
Figure 2. Comparison of healthcare costs to detected one affected pregnancy between base and reflex sgNIPT scenarios. Cost was calculated by dividing the total screening cost by the number of affected pregnancies detected.
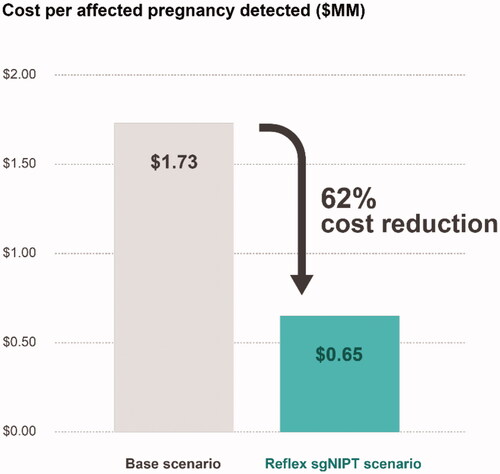
Table 4. Available prenatal and neonatal interventions for conditions screened by the reflex sgNIPT workflow.
Table 5. Total cost savings associated with SMA treatment selection per 100,000 pregnancies.
Figure 3. Sensitivity to inputs of reflex sgNIPT scenario cost savings per 100,000 pregnancies. The cost savings were calculated using a high and low value for each indicated input. The input value range is annotated next to each bar. The input values used in the model to yield $37.6 M in cost savings are as follows: % SMA Diagnosed Before Age 2 (Base) = 70%; % Eligible SMA Patients Choose Zolgensma = 70%; Paternal Follow-Up Rate = 41.5%; MFM Referral Rate (Base) = 36%; Paternal Testing Cost = $944; MFM Cost = $1,236; MFM Referral Rate (sgNIPT) = 80%; Diagnostic Testing Rate (sgNIPT) = 50%; Diagnostic Testing Cost = $730; Diagnostic Testing Rate (Base) = 50%.
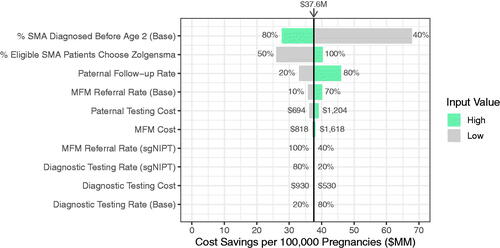
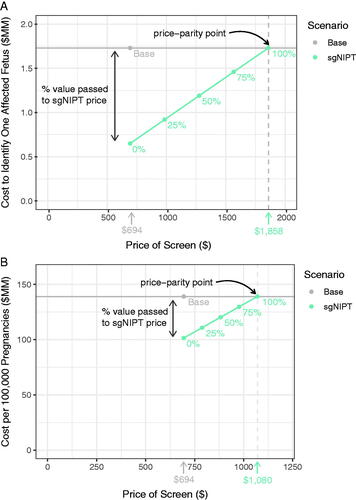
Figure 4. Pricing simulation for carrier screen with reflex sgNIPT using two different unit costs to determine the price-parity point with base scenario costs: (A) the cost to identify one affected fetus and (B) the cost per 100,000 pregnancies. Percentages refer to the percent of value (determined by the base costs) passed to the reflex sgNIPT costs.