Figures & data
Table 1. Eligibility criteria for identification of relevant studies.
Figure 1. PRISMA flow diagram. NAVIGATOR62 and SOURCECitation65 were additional trials to those identified through the systematic literature search. Abbreviations: CSR, clinical study report; HTA, Health Technology Assessment; MA, meta-analysis; NMA, network meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; SLR, systematic literature review.
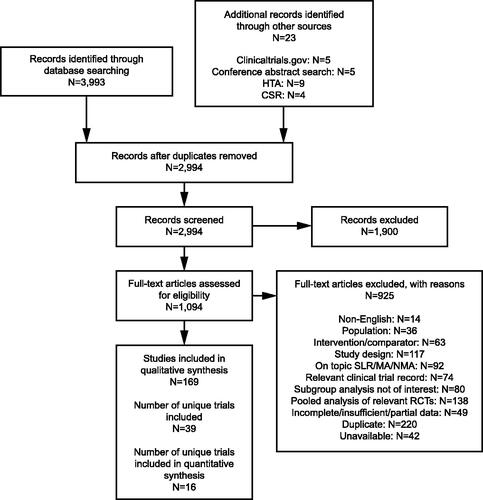
Figure 2. Network diagram for primary NMAs of AAER overall and AAER leading to hospitalization/emergency room visit. aThese studies were excluded from the sensitivity analysis that restricted inclusion to blinded and placebo-controlled trials. bThese studies were excluded from the sensitivity analysis that restricted inclusion to phase 3 and 4 studies. In this sensitivity analysis, studies were excluded if they were not phase 3 or 4 studies, or if their source article did not report the study phase. Castro et al.Citation8 reported data for two different studies (NCT01287039 and NCT01285323), so contributes two treatment arms. LIBERTY ASTHMA QUEST reported data for two different treatment arms, based on dupilumab dose. Abbreviations: AAER, annualized asthma exacerbation rate; NMA, network meta-analysis.
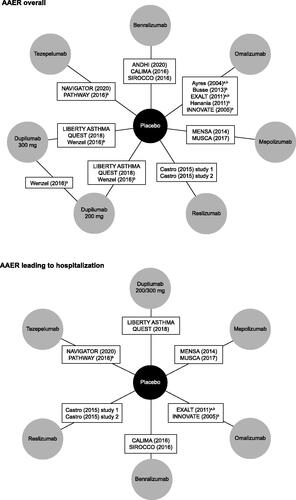
Table 2. Primary NMA: rate ratios for AAER overall, reported as cross-tabulation of all treatment arms in the network.
Table 3. Primary NMA: rate ratios for AAER leading to hospitalization/emergency room visit, reported as cross-tabulation of all treatment arms in the network.
Table 4. Subgroup NMA for patients with eosinophil count ≥ 300 cells/µL: rate ratios for AAER overall, reported as cross-tabulation of all treatment arms in the network.
Table 5. Subgroup NMA for patients with eosinophil count <300 cells/µL: rate ratios for AAER overall, reported as cross-tabulation of all treatment arms in the network.
Figure 3. Anchor and comparator trials used for the STC. Treatment effect of tezepelumab was simulated in the study population of each of the comparator trials. aCALIMA and SIROCCO were both suitable as comparator studies and had the same inclusion criteria; thus, these studies were pooled. bHanania et al.Citation55 was used for AAER overall because this study did not report results for AAER leading to hospitalization/emergency room visit. EXALT was used for AAER leading to hospitalization/emergency room visit. Abbreviations: AAER, annualized asthma exacerbation rate; STC, simulated treatment comparison.
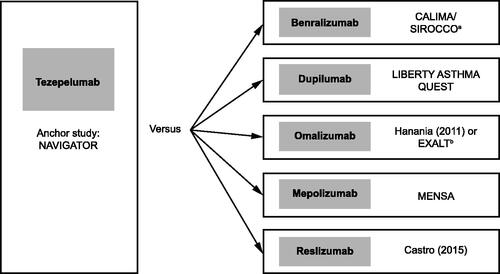
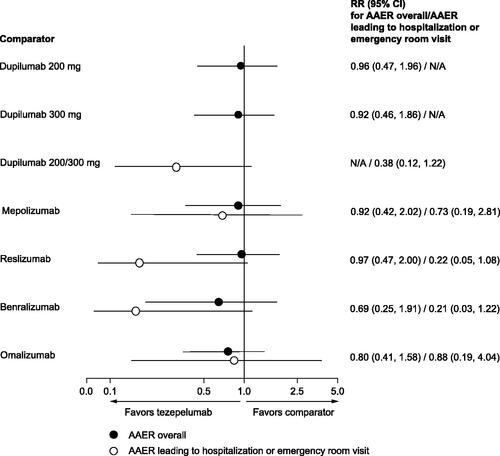
Figure 4. Findings from the STC, rate ratios for AAER, tezepelumab versus comparators. RR reflects the effect of tezepelumab in the comparator study population. Treatment effect modifiers included in the models of each comparator and outcome can be found in Supplementary Table S20. Abbreviations: AAER, annualized asthma exacerbation rate; CI, confidence interval; N/A, not applicable; RR, rate ratio; STC, simulated treatment comparison.