Figures & data
Figure 1. Constructed conditional survival curve for OS in the NIVO + IPI arm. Abbreviations. CM, CheckMate; IPI, ipilimumab; NIVO, nivolumab; OS, overall survival; PFS, progression-free survival; SEER, Surveillance, Epidemiology and End Results.
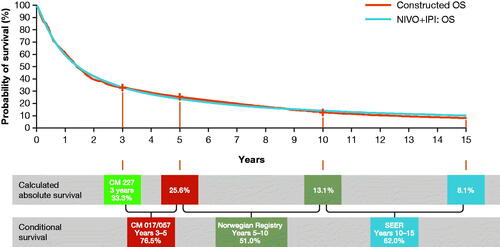
Figure 2. OS and PFS in the NIVO + IPI and PDC arms, comparing trial KM data and selected parametric curves. Abbreviations. IPI, ipilimumab; KM, Kaplan–Meier; NIVO, nivolumab; OS, overall survival; PDC, platinum-doublet chemotherapy; PFS, progression-free survival.
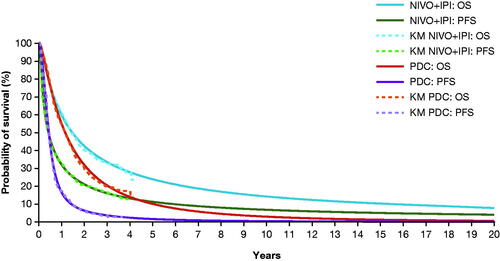
Table 1. Scenario analyses results for NIVO + IPI versus PDC.
Table 2. Model cost inputs.
Table 3. Base-case results for NIVO + IPI versus PDC.
Figure 3. Deterministic sensitivity analysis of NIVO + IPI versus PDC showing the 10 most impactful parameters on the model result. See Supplementary Material, Supplemental Methods, Sensitivity Analyses for details of the analysis. Abbreviations. ICER, incremental cost-effectiveness ratio; IPI, ipilimumab; NIVO, nivolumab; PDC, platinum-doublet chemotherapy; QALY, quality-adjusted life-year; TTD, time to death.
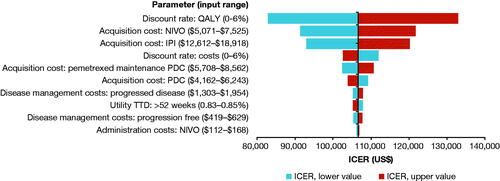
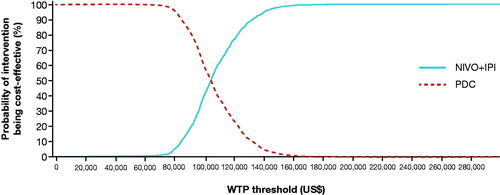
Figure 4. Cost-effectiveness acceptability curve for NIVO + IPI and PDC. See Supplementary Material, Supplemental Methods, Sensitivity Analyses for details of the analysis. Abbreviations. IPI, ipilimumab; NIVO, nivolumab; PDC, platinum-doublet chemotherapy; WTP, willingness to pay.
Supplemental Material
Download MS Word (99.2 KB)Data availability statement
The analysis reported in this study uses patient-level data from the CheckMate 227 trial. The results of the trial have been reported in a number of publicationsCitation18. The trial results supporting the findings of this analysis are presented graphically within the article. The survival analysis was implemented using the FlexSurv package in R.
