Figures & data
Figure 1. Sample attrition. Eligibility criteria along with stepwise sample attrition for the study is shown. Abbreviations. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; ATO, arsenic trioxide; ATRA, all-trans retinoic acid; HSCT, hematopoietic stem cell transplantation.

Table 1. Patient demographics.
Table 2. All-cause PPPM healthcare service utilization and costs over the follow-up period.
Figure 2. All-cause PPPM healthcare costs by duration of remission. All-cause PPPM healthcare costs are reported for the duration-of-remission subgroups over the full follow-up period and the mutually exclusive treatment, remission, and post-relapse periods. Costs are derived from paid amounts on administrative claims and adjusted to 2018 US dollars using the medical component of the Consumer Price Index. Sample size for the full follow-up, treatment, and remission periods: 0–<3 months, n = 256; 3–<6 months, n = 171; 6–<12 months, n = 119; and ≥12 months, n = 164. Sample size for the post-relapse period: 0–<3 months, n = 110; 3–<6 months, n = 90; 6–<12 months, n = 48; and ≥12 months, n = 19. Abbreviation. PPPM, per-patient-per-month.
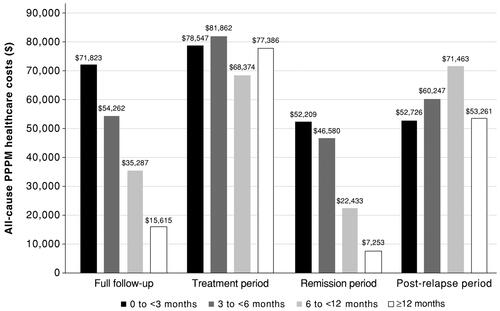
Table 3. Total all-cause healthcare costs for duration-of-remission subgroups by period.
Supplemental Material
Download MS Word (182.3 KB)Data availability statement
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
