Figures & data
Figure 1. Long-term survival and time-on-treatment extrapolations for pembrolizumab regimens and comparators in patients with CPS ≥1 (left column) and in overall R/M HNSCC population (right column). Abbreviations. CPS, combined positive score; EXTREME regimen, Cetuximab + platinum + 5-Fluorouacil; KM, Kaplan-Meier; OS, overall survival; PF, progression-free; PFS, progression-free survival; TPEx regimen, Cisplatin + docetaxel + cetuximab; 5-FU, 5-fluorouracil.
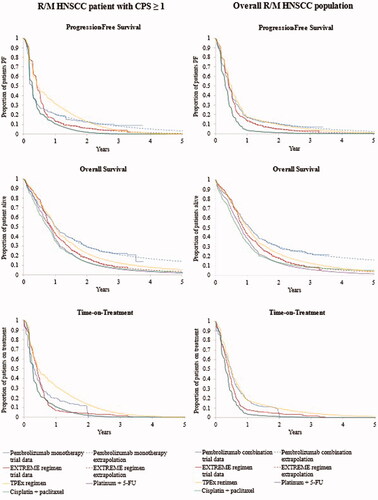
Table 1. Drug regimens and the calculated average cost per week.
Table 2. Model inputs for direct medical costs and health utility.
Table 3. Base-case cost-effectiveness analysis results for first-line pembrolizumab vs. comparators in R/M HNSCC over a 20-year time horizon.
Figure 2. The main variation in ICER of pembrolizumab regimens vs. the EXTREME regimen trial comparator upon one-way deterministic changes in model parameters. Abbreviations. CPS, combined positive score; EXTREME regimen, Cetuximab + platinum+5-Fluorouacil; PD, progressive disease; PF, progression-free; PFS, progression-free survival; QALY, quality-adjusted life years; R/M HNSCC, recurrent or metastatic head and neck squamous cell carcinoma; 5-FU, 5-fluorouracil.
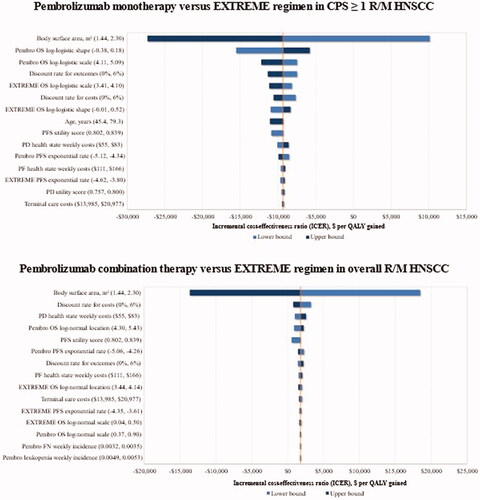
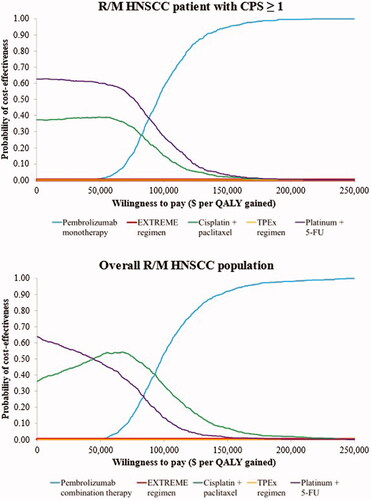
Figure 3. Cost-effectiveness acceptability curves for pembrolizumab monotherapy and comparators in R/M HNSCC patients with CPS ≥1 and pembrolizumab combination and comparators in the overall R/M HNSCC population. Abbreviations. CPS, combined positive score; EXTREME regimen, Cetuximab + platinum+5-Fluorouacil; QALY, quality-adjusted life years; R/M HNSCC, recurrent or metastatic head and neck squamous cell carcinoma; TPEx regimen, Cisplatin + docetaxel + cetuximab; 5-FU, 5-fluorouracil.