Figures & data
Table 1. Baseline demographics and disease burden.
Figure 1. Mean change from baseline in the number of migraine headache days with acute medication use. Abbreviations: GMB, galcanezumab; LS, least squares; OLE, open-label extension; SE, standard error. *p < .05 vs previous placebo. ***p < .001 vs placebo.
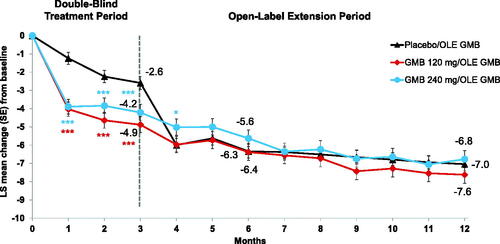
Figure 2. Acute medication overuse by treatment group. Abbreviations: GMB, galcanezumab; OLE, open-label extension. ***For Month 1, Month 2, and Month 3 (Double-blind period), galcanezumab rates vs placebo were statistically significantly lower (p < .001).
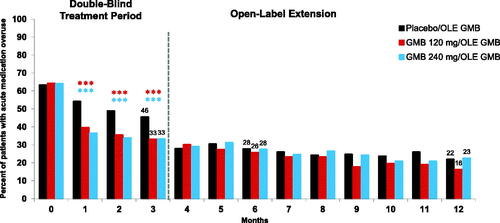
Figure 3. Migraine-specific healthcare resource utilization as count of patients with at least 1 visit (%) during treatment period. (a) Healthcare Professional Visits; (b) Emergency Room Visits; (c) Hospital Admissions. Abbreviations: GMB, galcanezumab; OLE, open-label extension period. aThe reported baseline value is the collected baseline value (previous 6 months) divided by 2.
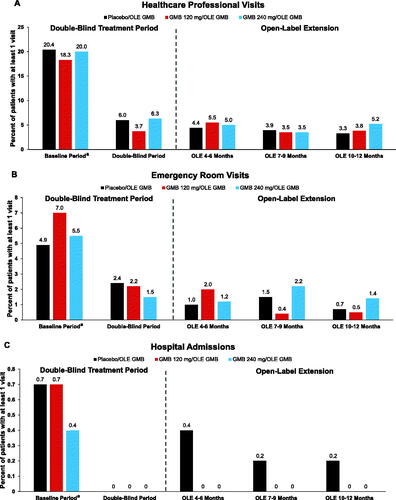
Table 2. Migraine-specific healthcare resource utilization rates per 100 patient-yearsa.
Data sharing statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
