Figures & data
Table 1. Model and scenario analyses inputs.
Figure 1. Five-year costs associated with using rFVIIIFc and emicizumab for prophylactic treatment of 100 people with hemophilia A (base-case analysis: recommended wastage; emicizumab 1.5 mg/kg once weekly). (a) Adolescents/adults (≥12 years). (b) Children (<12 years). Abbreviation: rFVIIIFc, recombinant factor VIII Fc.
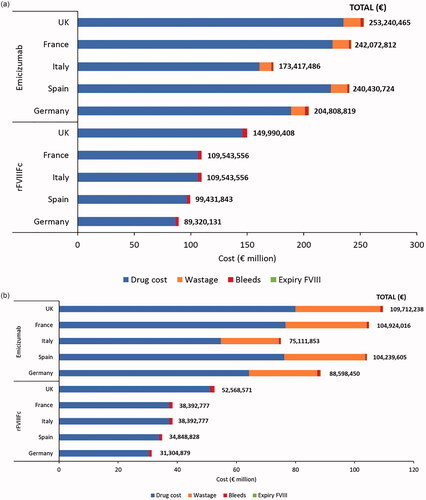
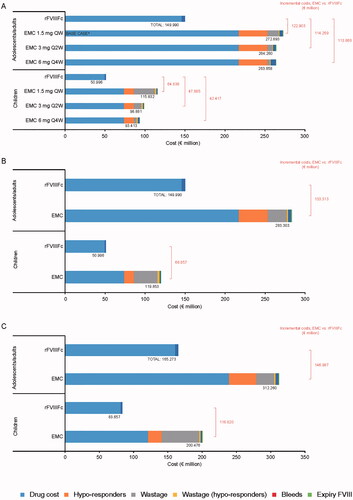
Figure 2. Scenario analyses (UK): 5-year costs associated with using rFVIIIFc and emicizumab for prophylactic treatment of 100 people with hemophilia A, according to (a) Reduced dosing frequency for emicizumab, (b) Emicizumab potential maximum wastage, and (c) Maximal emicizumab wastage according to bodyweight. (a) Base-case dosing interval for emicizumab (1.5 mg every week) included for comparative purposes. (b) Elocta dose set at 88.11 IU/kg/week. Abbreviations: QW, once weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; rFVIIIFc, Recombinant factor VIII Fc.
Supplemental Material
Download MS Word (61.3 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author [GC], upon reasonable request.
