Figures & data
Figure 1. Schematic overview of the ACCORDANT study. ACCORDANT subjects (N = 847) were routed to three study arms: standard care (SoC) control; risk predictor plus case management intervention (RP-CM); or risk predictor plus multimodal management intervention (case management with pharmacological treatment) (RP-MM). Those in the RP-CM and RP-MM arms were stratified into higher-risk and lower-risk groups based on their proteomic biomarker risk predictor scores, using a previously validated threshold. Subjects predicted to be at higher preterm birth risk were modeled to receive case management or multimodal management. Lower-risk subjects in the RP-CM and RP-MM arms received standard care. The gestational ages at birth of higher-risk subjects were shifted according to published intervention efficacies. Associated outcomes were calculated for the treated groups and for subjects with missing or truncated data in both arms. Outcomes were compared between active arms and the control arm, as a whole and between the most severely affected 10% of each arm by outcome.
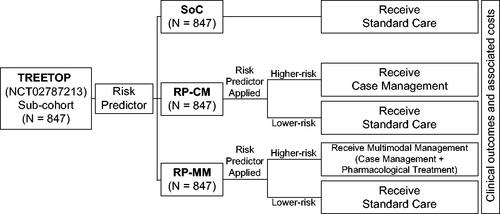
Figure 2. Shifts in gestational ages at birth applied to subjects who were predicted to be at higher risk for preterm birth and subsequently modeled to receive case management (increased outreach, preterm education and specialist care) or multimodal management (case management with pharmaceutical treatment). Shifts were based on published intervention efficacies.
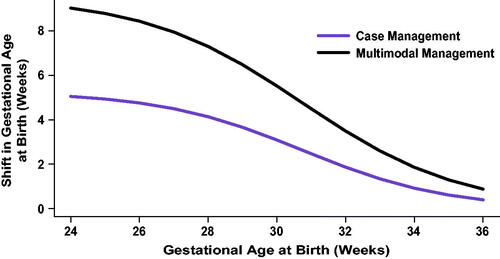
Table 1. Summary of ACCORDANT assumptions.
Figure 3. Impact of test-and-treat strategies on neonatal length of hospital stay. The population assessed was either (A) all subjects or (B) those in the top 10% with respect to length of stay. *p < .05, determined using Cox proportional hazards regression. **p < .001.
Abbreviations. COM, U.S. commercial payer dataset; MIX, U.S. state-based dataset with a mix of insurance payers.
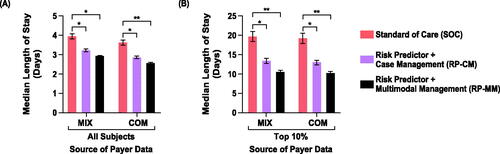
Figure 4. Impact of test-and-treat strategies on neonatal length of hospital stay (A,B) and neonatal costs (C,D) among self-identified Black and Hispanic individuals. The population assessed was either all such subjects (A,C) or those in the top 10% with respect to length of stay or costs (B,D). Abbreviations. COM, U.S. commercial payer dataset; MIX, U.S. state-based dataset with a mix of insurance payers.
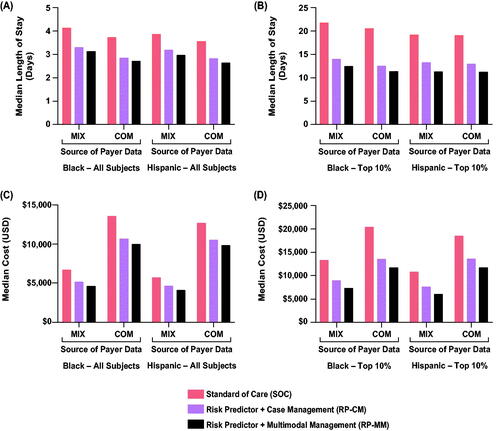
Figure 5. Impact of test-and-treat strategies on neonatal costs. The population assessed was either (A) all subjects or (B) those in the top 10% with respect to costs. *p < .05, determined using bootstrap intervals. Abbreviations. COM, U.S. commercial payer dataset; MIX, U.S. state-based dataset with a mix of insurance payers; ns, non-significant.
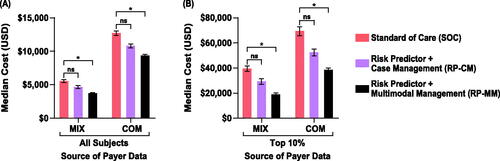
Table 2. Impact of test-and-treat strategies on neonatal morbidity and mortality.
Supplemental Material
Download PDF (540.6 KB)Data availability statement
Data supporting the results presented here can be requested by emailing the corresponding author. Data will not be made available publicly or in any format that may violate a subject’s right to privacy.
