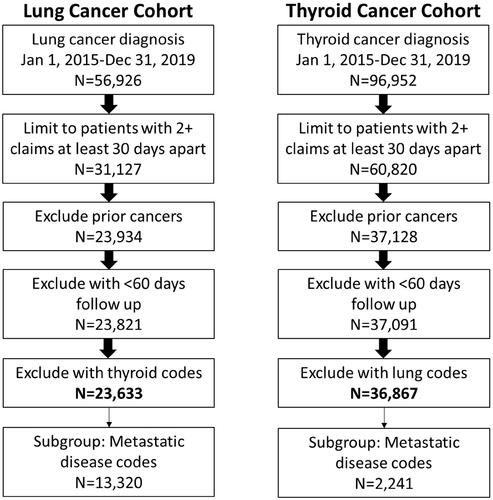
Figures & data
Table 1. Patient characteristics at diagnosis.
Table 2. Characteristics of patients with lung cancer, with and without biomarker testing codes.
Table 3. Characteristics of patients with thyroid cancer, with and without biomarker testing codes.
Table 4. Median (interquartile range, IQR) and mean (standard deviation, SD) lifetime per-patient payer costs of biomarker testing, by payer and tumor type.
Table 5. Median and interquartile range (IQR) costs of single-gene and comprehensive/NGS-based testing codes.
Data availability statement
Data used in this study were supplied by International Business Machines Corporation under license for scientific research at Eli Lilly and Company. Data are available for research purposes by licensing directly from IBM, Inc. (www.ibm.com/products/marketscan-research-databases).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
A poster containing an earlier version of data from this study was presented at ISPOR International Conference (Washington, DC) on 15–18 May 2022.