Figures & data
Figure 1. Diagram of the Markov model. DFS, disease-free survival; eNSCLC, early non-small cell lung cancer; 1L, first line; 2L, second line.
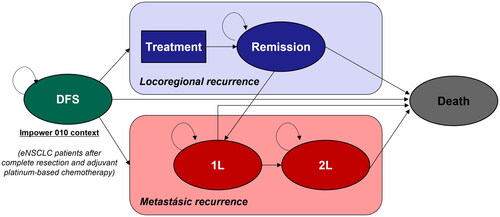
Table 1. Transition probabilities.
Table 2. Treatments distribution in each health state.
Table 3. Base case results.
Figure 2. Tornado diagram. DFS, disease-free survival; ICUR, incremental cost-utility ratio; Ind, indirect costs; IV, intravenous; QALY, quality-adjusted life year; 1L, first line; 2L, second line.
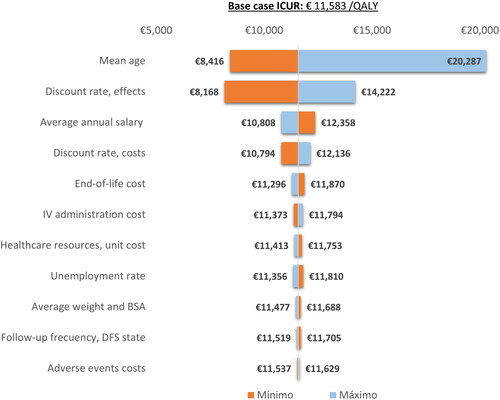
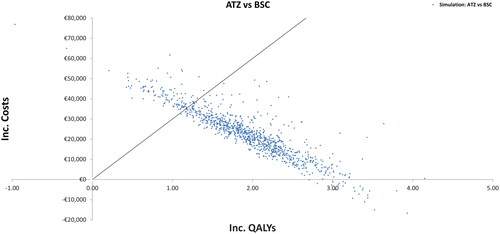
Figure 3. Incremental cost-effectiveness plane. ATZ, atezolizumab; BSC, best supportive care; Inc, incremental; QALYs, quality-adjusted life years
Supplemental Material
Download MS Word (59 KB)Data availability statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
