Figures & data
Figure 1. Base-case estimated incidence per 100,000 person-years of A) cervical cancer, B) all other cancers (female), and C) all cancers (male) associated with human papillomavirus strains covered by the nonavalent vaccine.
‘Male’ and ‘female’ refer to sex assigned at birth. FOV, female-only vaccination with coverage rates of 30% among the primary cohort and 15% among the catch-up cohort; GNV, gender-neutral vaccination with coverage rates of 30% among the primary female cohort and 15% among the female catch-up and male cohorts.
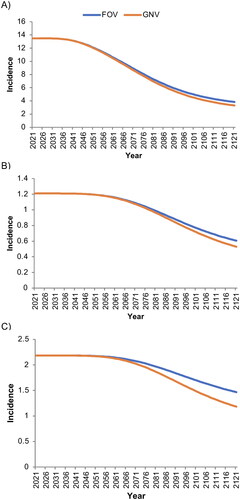
Figure 2. Base-case estimated incidence per 100,000 person-years of A) anogenital warts (female), B) anogenital warts (male), C) Juvenile-Onset recurrent respiratory papillomatosis (female), and D) Juvenile-Onset recurrent respiratory papillomatosis (male) associated with human papillomavirus strains covered by the nonavalent vaccine.
‘Male’ and ‘female’ refer to sex assigned at birth. FOV, female-only vaccination with coverage rates of 30% among the primary cohort and 15% among the catch-up cohort; GNV, gender-neutral vaccination with coverage rates of 30% among the primary female cohort and 15% among the female catch-up and male cohorts.
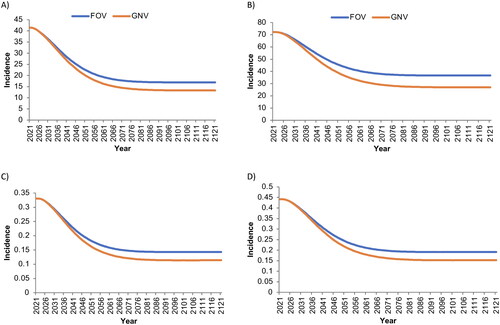
Table 1. Cases and deaths averted in the base-case analysis for gender-neutral instead of female-only human papillomavirus vaccination strategies over 100 years in Japan.
Table 2. Base-case health care costs and quality-adjusted life years lost for gender-neutral versus female-only vaccination strategies over 100 years in Japan.
Figure 3. Tornado diagram illustrating how varying key model parameters affects the incremental cost-effectiveness ratio in the base case.
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years.
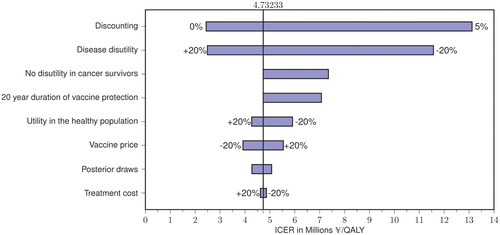
Table 3. One-way sensitivity analyses to determine how key model parameters affect the incremental cost-effectiveness ratio of gender-neutral versus female-only vaccination over 100 years in the base case.
Table 4. Public health and cost-effectiveness outcomes for gender-neutral versus female-only human papillomavirus vaccination strategies over 100 years in scenario analyses with varying female, catch-up, and male vaccination coverage rates.
