Figures & data
Figure 1. Model structure. Note: Patients’ distribution among health states over time were estimated using the fitted PFS and OS curves. At each model cycle, the proportion of patients remaining alive and discontinuing treatment were estimated by means of TTOT data from the GO29871 study for mosunetuzumab whereas for all other treatments, the TTOT is set equal to the selected parametric distribution for PFS, capped at the treatment-specific maximum number of cycles ascertained from US PIs. Abbreviations. OS, overall survival; PIs, package inserts; PFS, progression-free survival; PPS, post-progression disease; TTOT, time-to-off treatment.
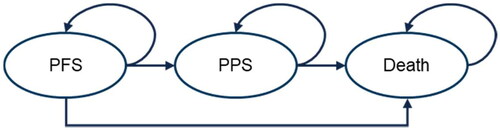
Table 1. Model parameters.
Figure 2. Net monetary benefit associated with mosun (WTP per QALY of $150,000). Abbreviations. Axi-cel, axicabtagene ciloleucel; Mosun, mosunetuzumab; O-Benda, obinutuzumab + bendamustine; QALY, quality-adjusted life year; R-Benda, rituximab + bendamustine; R-Len, rituximab + lenalidomide; RW, real-world cohort; Taz, tazemetostat; Tisa-cel, tisagenlecleucel; vs., versus; WTP, willingness-to-pay.
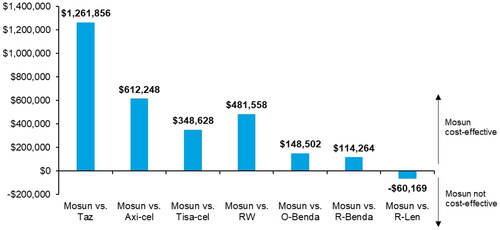
Table 2. Cost-effectiveness results in base case analysis: costs, life-years, quality-adjusted life-years, and ICERs.
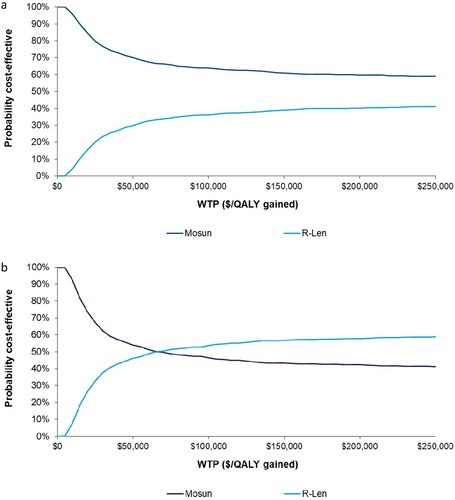
Figure 3. (a) CEAC of mosun vs. R-Len using alternative OS parametric curve (Log-logistic). Abbreviations: Mosun, mosunetuzumab; R-Len, rituximab + lenalidomide; QALY, quality-adjusted life year; WTP, willingness-to-pay. (b) CEAC of mosun vs. R-Len using base case OS parametric curve (exponential). Abbreviations. Mosun, mosunetuzumab; R-Len, rituximab + lenalidomide; QALY, quality-adjusted life year; WTP, willingness-to-pay.
Supplemental Material
Download MS Word (564 KB)Data availability statement
The data that support the findings of this study are available in this article. The model is not publicly available due to its intrinsic commercial value and cannot be shared for legal reasons.
