Figures & data
Figure 1. Study design.
Abbreviations: HCPs, healthcare providers; PFS, prefilled syringe; Vaccine A, ready-to-use PFS; Vaccine B, VRR1 (vial with a lyophilized vaccine and vial with an adjuvant suspension); Vaccine C, VRR2 (vial with lyophilized vaccine and a prefilled syringe with sterile water diluent); VRR, vaccine requiring reconstitution; VRR1, VRR with vial with a lyophilized vaccine and vial with an adjuvant suspension; VRR2, VRR with vial with lyophilized vaccine and a prefilled syringe with sterile water diluent.
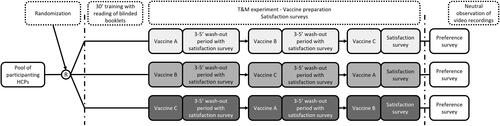
Table 1. Participant demographics, overall and by randomization sequence.
Table 2. Vaccine preparation time, by vaccine and for pooled VRRs, with comparison.
Table 3. Vaccine preparation errors, by vaccine and for pooled VRRs.
Figure 3. Participant satisfaction ratings for (a) ease of preparation, (b) time of preparation, and (c) overall satisfaction with vaccine preparation.
Abbreviations: PFS, ready-to-use prefilled syringe; VRR, vaccine requiring reconstitution; VRR1, VRR with vial with a lyophilized vaccine and vial with an adjuvant suspension; VRR2, VRR with vial with lyophilized vaccine and a prefilled syringe with sterile water diluent.
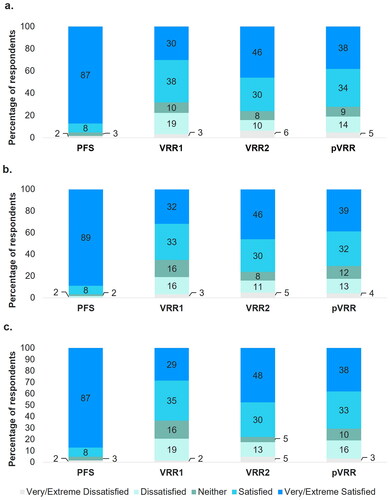
Table 4. Comparison of participant satisfaction with vaccine preparation procedures.
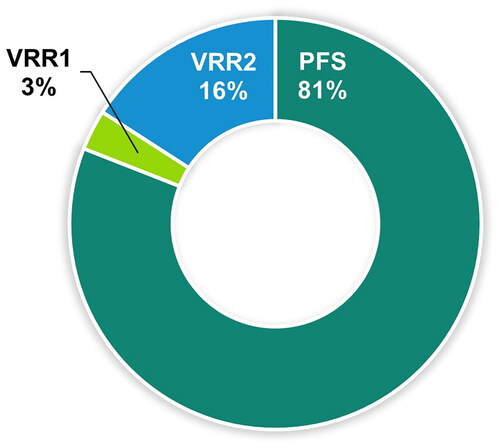
Figure 4. Participant preference for vaccine preparations.
Abbreviations: PFS, ready-to-use prefilled syringe; VRR, vaccine requiring reconstitution; VRR1, VRR with vial with a lyophilized vaccine and vial with an adjuvant suspension; VRR2, VRR with vial with lyophilized vaccine and a prefilled syringe with sterile water diluent.
Supplemental Material
Download MS Word (84.6 KB)Data availability statement
All data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.