Figures & data
Table 1. Demographics and clinical characteristics of people with CFTable Footnotea in 2019 (n = 743).
Figure 1. Mean HCRU in 2019 for people with CF compared with the general population. (a) Number of outpatient specialist visits. (b) Number of hospitalizations. (c) Number of hospitalization days. (d) Number of CF-related dispensations. (e) Number of days’ work absence. (f) Number of VAB leave days. Values graphed are means. Deltas (Δ) indicate differences in the means, which are followed by the 95% confidence intervals. The number of people with CF included in the analyses were: 743 of any age, 270 children aged <18 years, 473 adults aged ≥18 years, 18 children aged <2 years, 159 children aged 2 to <12 years, and 93 children aged 12 to <18 years. The number of people in the control groups were: 7406 of any age, 2700 children aged <18 years, 4706 adults aged ≥18 years, 180 children aged <2 years, 1590 children aged 2 to <12 years, and 930 children aged 12 to <18 years. CF-related dispensations included adrenergic inhalators/bronchodilators, antibiotics, anticoagulants, anticonvulsants, antidepressants, antidiabetics, antifungals, antihistamines, anti-inflammatory agents, anxiolytics, corticosteroids, expectorant drugs, gastroesophageal reflux disease medications, growth hormones, opioids, osteoporosis drugs, oxygen, pancreatic enzymes, transplant immunosuppressants, and vitamins. CF, cystic fibrosis; HRCU, healthcare resource utilization; VAB, care of sick child (vård av barn).
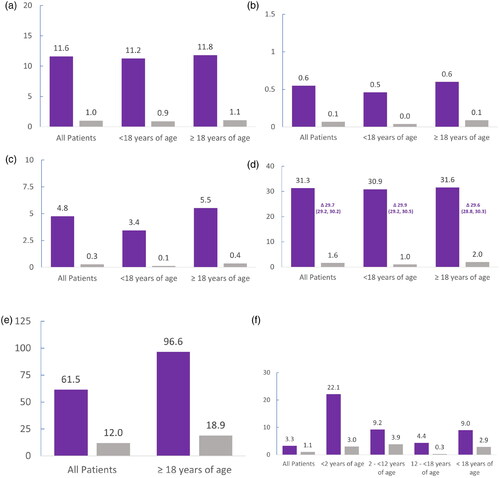
Table 2. Comparison of total costs (€ 2020) in people with CF and controls in 2019Table Footnotea.
Table 3. Impact of treating with LUM/IVA on clinical outcomesTable Footnotea.
Table 4. Impact of treatment with LUM/IVA on resource use and costsTable Footnotea.
Supplemental Material
Download MS Word (78.2 KB)Data sharing statement
Each registry holder collects and manages its own data and maintains processes for researchers to request data. Restrictions apply to the sharing of these data, which were accessed for the purposes of the study based on an ethics approval and individual agreements with each respective registry holder.
