Figures & data
Table 1. Crystallization solvents, melting points, yield percentages, molecular formulas, and molecular weights of compounds 4–24.
Scheme 1. Reagents: a, CS2/KOH; b,(CH3)2SO4; c, RNCS/C2H5OH; d, ArCOCH2X/(CH3)2CHOH; e, C2H5ONa/C2H5OH; f, NH2NH2.H2O/C2H5OH; g, RCOC1/pyridine.
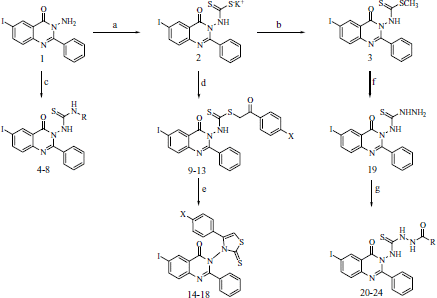
Table 2. Antimicrobial activity of compounds (200 μ g/8 mm disk), the broad-spectrum antibacterial drug ampicillin trihydrate (100 μ g/8 mm disk) and the antifungal drug Clotrimazole (100 μ g/8 mm disk) against S. aureus ATCC 19433 (SA), B. subtilis ATCC 6633 (BS), E. coli ATCC 25922 (EC), P. aeruginosa ATCC 27853 (PA), and C. albicans ATCC 753 (CA).
Table 3. The minimal inhibitory concentrations (MIC, μg/mL) of indicated compounds, the broad spectrum antbacterial drug ampicillin trihydrate, and the antifungal drug clotrimazole against S. aureus ATCC 19433 (SA), B. subtilis ATCC 6633 (BS), E. coli ATCC 25922 (EC), P. aeruginosa ATCC 27853 (PA) and C. albicans ATCC 753 (CA).