Figures & data
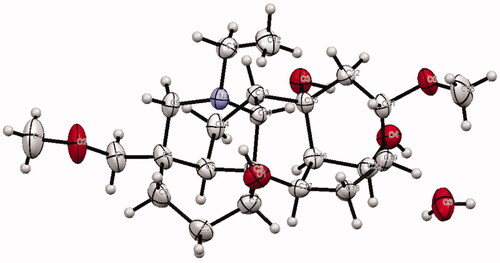
Table 1. Data related to structure determination and refinement of compound 1.
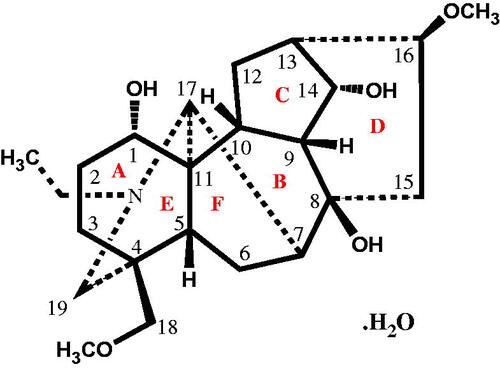
Table 2. List of selected bond lengths (Å) and bond angle (o) of compound 1.
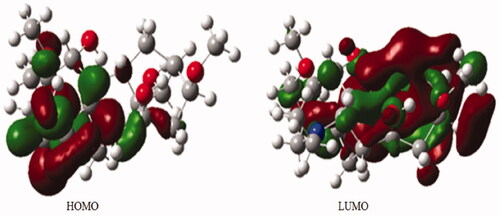
Table 3. Energy parameters of the compound 1.
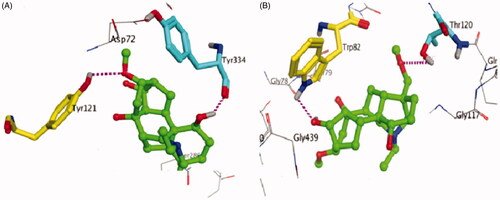
Figure 3. (A) Acetylecholinesterase inhibition by compound 1 is the Lineweaver–Burk plot of reciprocal of initial velocities versus reciprocal of four fixed substrate concentrations in absence (•) and presence of 100 μM (▪), 75 μM (▴), 50 μM (×) of compound 1. (B) Butyrylecholinesterase inhibition by 1 in absence (•) and presence of 100 μM (▪), 75 μM (▴), 50 μM (×) of compound 1.
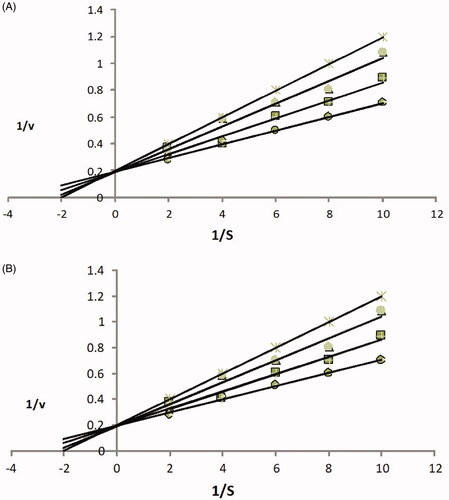
Table 4. AChE and BChE inhibitory activities of compound 1 (isotalatizidine hydrate).