Figures & data
Table 1. Total phenolic and total flavonoid contents of cactus cladode extract.
Table 2. Reducing power and Fe2+ chelating activity of cactus cladode extract.
Table 3. Change in hematological parameters of control and rats treated with lithium carbonate (Li), CCE or their combination (CCE + Li).
Table 4. Assay of serum markers of control and rats treated with lithium carbonate (Li), CCE, or their combination (CCE + Li).
Figure 1. HPLC profile of phenolic acids (λ = 280 nm) from cactus (Opuntia ficus-indica) cladodes (CCE). Six known phenolic acids identified in CCE: gallic acid (1), catechin (4), caffeic acid (5), epicatechin (6), vanillic acid (7) and coumarin (8). The HPLC separation of the active compounds was carried out on C-18 reverse phase HPLC column (Zorbax, 250 mm ×4.6 mm, particle size 5 μm) on an elution gradient at 25 °C. The mobile phase consisted of water:acetic acid (98:2 v/v) (A) and water:acetonitrile:acetic acid (58:40:2 v/v/v) (B). The elution gradient used was: 0–80% B for 25 min, 80–100% B for 10 min and 100–0% B for 5 min. The flow rate was 0.9 mL/min and the injection volume was 20 μL.
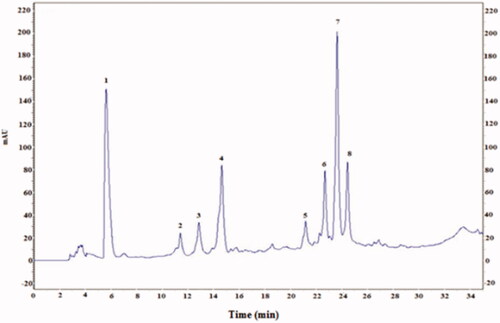
Figure 2. HPLC profile of flavonoids (λ = 360 nm) from cactus (Opuntia ficus-indica) cladodes (CCE). Four known flavonoids identified in CCE: rutin (2), isorhamnetin (3), quercetin (6), kampferol (7). The HPLC separation of the active compounds was carried out on C-18 reverse phase HPLC column (Zorbax, 250 mm ×4.6 mm, particle size 5 μm) on an elution gradient at 25 °C. The mobile phase consisted of water:acetic acid (98:2 v/v) (A) and water:acetonitrile:acetic acid (58:40:2 v/v/v) (B). The elution gradient used was: 0–80% B for 25 min, 80–100% B for 10 min and 100–0% B for 5 min. The flow rate was 0.9 mL/min and the injection volume was 20 μL.
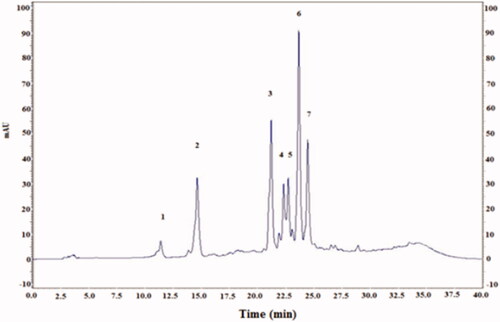
Figure 3. Infrared spectra of the polysaccharide extracted from CCE recorded in the frequency range of 4000–500 cm−1.
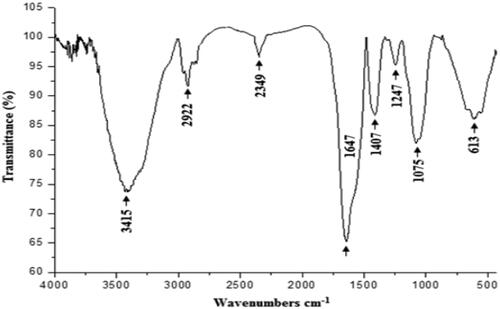
Figure 4. DPPH-radical-scavenging activity of CCE and positive control BHT at different concentrations. Data are expressed as mean ± standard deviation of the mean (n = 3).
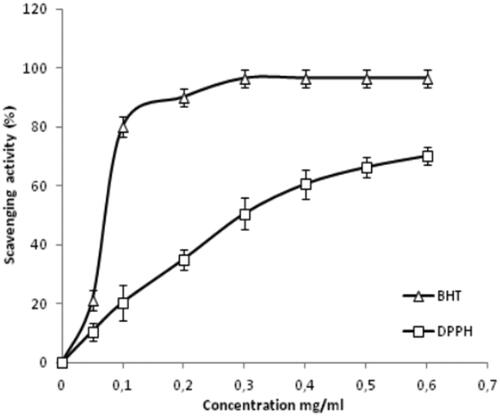
Figure 5. Effect of lithium carbonate and CCE on MDA level and antioxidant enzyme activities (SOD, CAT and GPx) in control (C), carbonate lithium-treated (Li), cactus-treated (CCE), and cactus supplemented with carbonate lithium (Li + CCE) groups. Data are expressed as means ± SD for six rats in each group. Statistical comparison was performed using Student’s t-test. *p < 0.05, **p < 0.01 compared with control group (C). +p < 0.05, ++p < 0.01 compared with lithium carbonate (Li)-treated group.
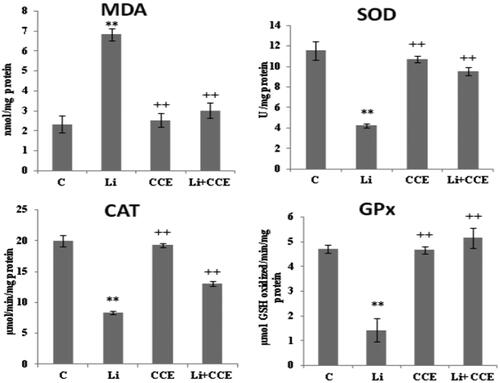
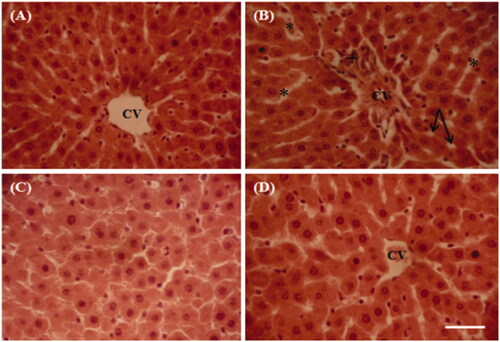
Figure 6. Representative photographs from the liver showing the protective effect of cactus cladode extract on lithium carbonate induced hepatic injury in rats. (A) Controls, (B) rats treated with lithium carbonate, (C) rats treated with cactus cladode extract and (D) rats treated with the combination of cactus cladode extract and lithium carbonate. Liver sections were stained using the haematoxylin-eosin method. Original magnifications: ×400; CV: central vein in the liver + congested central veins; *sinusoidal dilatation; vacuolization: inflammatory cell infiltration.