Figures & data
Figure 1. Data of microscopic evaluations (histology) of skin from the tails of rats with radiation-induced wounds that were treated with 50 mg/mL of AEE or EAE from the fruit of D. indica L. and the control groups. The analysis was performed in technical triplicates with samples of tissue of six mice from each group (biological replicates). The positive control was treated with clobetasol propionate (0.5 mg/mL), and the negative control was treated only with the excipient (1 mL of ethanol:water 1:2). (A) Percentage of parakeratosis compared to orthokeratosis and (B) quantification of infiltrating inflammatory cells. (C) Image of a slice of tissue from a positive control showing no parakeratosis (score 1) and, predominant orthokeratosis instead. (D) Images of tissues used to grade parakeratosis in comparison to orthokeratosis: minimum (score 2), (E) moderate (score 3). (F) Image of a slice of tissue from a negative control with intense parakeratosis (score 4) and swollen epidermal cones with inflammatory infiltrate. 400 × magnification (*) denotes significant difference compared to the negative control, p < 0.001. (#) denotes significant difference compared to AEE treatment, p < 0.05.
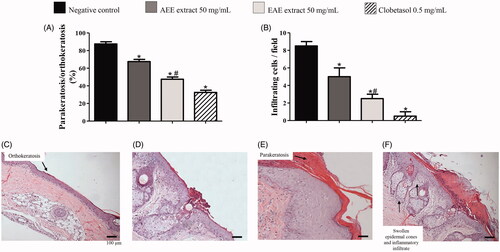
Table 1. Features of the extraction procedures and data of standardization of extracts to betulinic acid.
Figure 2. HPLC chromatograms of betulinic acid (0.2 mg/mL) of (A) analytical standard grade, (B) the AEE at 5 mg/mL and (C) the EAE at 10 mg/mL with peaks corresponding to betulinic acid at 4.6 min. (D) Phytochemical screening of AEE and EAE, where (+/++/+++) corresponds to light, moderate and intense positive reactions (presence of the constituent), respectively, and (−) corresponds to a negative reaction (absence of the constituent).
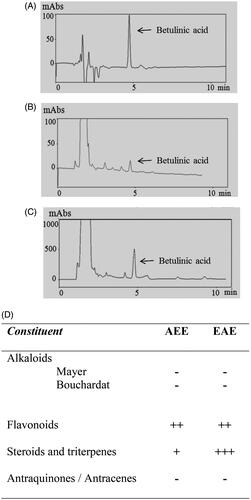
Figure 3. Prevention of lipid peroxidation in vitro by the AEE and the EAE from the fruit of D. indica. (*) denotes significant difference compared to the control, in which egg yolk lipids were treated with the free radical-generating compound 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), p < 0.001. Data are the means of three independent experiments.
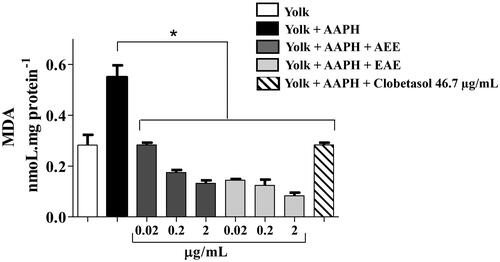
Figure 4. (A–F) The wound-healing effect of AEE and EAE from the fruit of D. indica applied topically on radiation-induced psoriasis-like wounds in rats and the control groups. Closed bars correspond to wounds during the repair process, and open bars correspond to completely healed wounds. The negative control was treated only with (A) the excipient (1 mL of ethanol:water 1:2), and the positive control was treated with (B) clobetasol propionate at 0.5 mg/mL. AEE and EAE were applied at (C/D) 5 mg/mL and (E/F) 50 mg/mL, respectively. The evolution of wound healing in the tails of rats treated with EAE at (G) 50 mg/mL; wounds healed on day 14 in this group. (*) denotes significant difference compared to the negative control, p < 0.001. (#) denotes significant difference compared to AEE treatment at 50 mg/mL, p < 0.05. Data are means of six mice from each group (biological replicates).
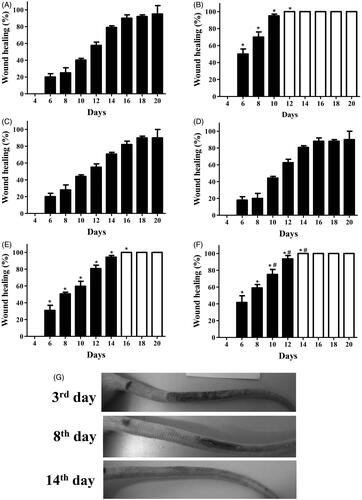
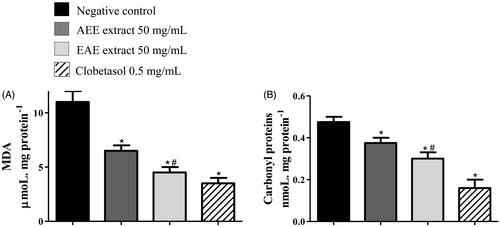
Figure 5. Protection against oxidative damage in the tail skin of rats with radiation-induced wounds that were treated with 50 mg/mL of AEE or EAE from the fruit of D. indica and the control groups. The positive control was treated with clobetasol propionate (0.5 mg/mL), and the negative control was treated only with the excipient (1 mL of ethanol:water 1:2). The analysis was performed in technical triplicates with tissue samples of six mice from each group (biological replicates). Protection against (A) lipid peroxidation and (B) protein carbonylation. (*) denotes significant difference compared to the negative control, p < 0.001. (#) denotes significant difference compared to AEE treatment, p < 0.05.