Figures & data
Table 1. 1H (500 MHz) and 13C (125 MHz) NMR data of compounds 1, 4, and 5 [δ (ppm), J (Hz)].
Figure 1. Chemical structures of isolated compounds (1–20): oleanolic acid (1), betulinic acid (2), pomolic acid 3β-acetate (3), rotungenic acid (4), rotundic acid (5), stigmast-4-ene-3,6-dione (6), daucosterol (7), benzyl hydroperoxide (8), 2,3-dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-propan-1-one (9), octadeca-9Z,12Z-dienoic acid (10), 9,12,13-trihydroxyoctadeca-10E-enoic acid (11), 9,12,13-trihydroxyoctadeca-10E,15Z-dienoic acid (12), α-tocopherolquinone (13), (7S*,8R*,7′R*,8′S*)-icariol A2-9-O-β-xylopyranoside (14), (2S,3S,4R,2′R)-2-(2′-hydroxytetracosanoylamino)octadecane-1,3,4-triol (15), (2S)-1-O-linolenoyl-2-O-linolenoyl-3-O-β-d-galactopyranosyl-sn-glycerol (16), (2S)-1-O-linolenoyl-2-O-linoleoyl-3-O-β-d-galactopyranosyl-sn-glycerol (17), (2S)-1-O-linoleoyl-2-O-linoleoyl-3-O-β-d-galactopyranosyl-sn-glycerol (18), (2S)-1-O-linolenoyl-2-O-palmitoyl-3-O-β-d-galactopyranosyl-sn-glycerol (19), and (2S)-1-O-linoleoyl-2-O-palmitoyl-3-O-β-d-galactopyranosyl-sn-glycerol (20).
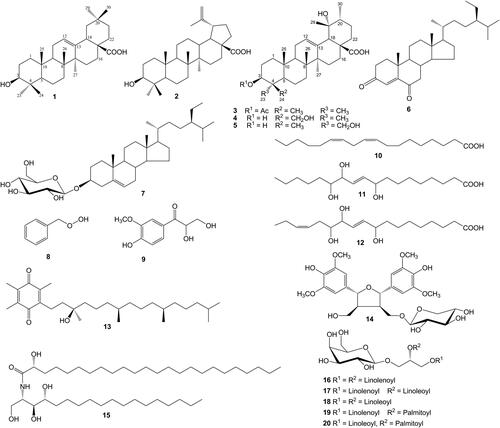
Table 2. Antibacterial activities of compounds 1, 2, 4–6, and 15.
