Figures & data
Table 1. Results of biochemical analysis performed on rat serum subjected to different treatments at 34th weeks post injection of DENA.
Table 2. Results of biochemical analysis performed on rat sera subjected to different treatments at 38th weeks post injection of DENA.
Table 3. Results of MDA concentration and SOD activity at the 34th and 38th week post injection of DENA.
Figure 1. Livers, rats (38th week). (a) Normal hepatic structure in group I (control group). (b) Hepatocellular adenoma in group V injected with DENA. (c) Altered hepatocellular focus with decreased cellular density in group VI injected with DENA and treated with camel milk. (d) Hepatocellular adenoma with spongiosis hepatis in group VII injected with DENA and treated with cisplatin. (e) Mononuclear inflammatory cells invading the periphery of altered hepatocellular focus and single cell necrosis in group VI injected with DENA and treated with camel milk and cisplatin. Haematoxylin and eosin stain 200×.
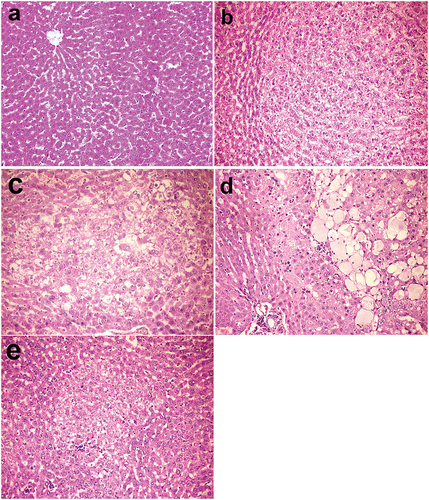
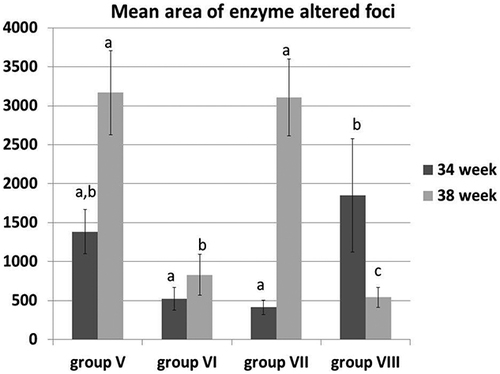
Figure 2. The mean area of altered hepatocellular foci in different groups at 34th and 38th week. All data were presented as mean value (n = 10) ± standard error. Values bearing different superscripts (a, b, c) are significant at p < 0.05.
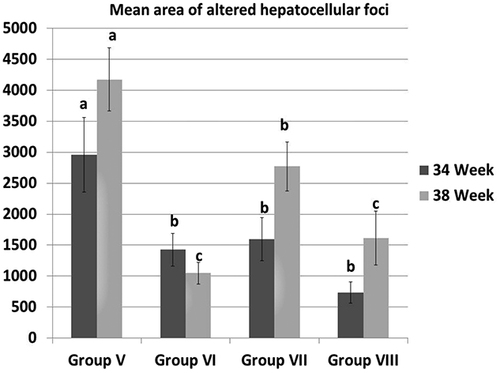
Figure 3. Kidneys, rats (38th week). (a) Normal renal tissue in group I control group. (b) Tubular cell adenoma of solid pattern with compression of adjacent parenchyma in group V injected with DENA. (c) Minor histopathological alteration in group VI injected with DENA and treated with camel milk. (d) Mononuclear inflammatory cells infiltration in the interstitial tissue with thickening of glomerular and tubular basement membrane and fibroplasia in group VII injected with DENA and treated with cisplatin. (e) Bluish-stained periglomerular and interstitial fibroplasia (Massons’ trichrome stain). (f) Few mononuclear inflammatory cells infiltration with regenerated renal tubules in group VI injected with DENA and treated with camel milk and cisplatin. Haematoxylin and eosin stain 200×.
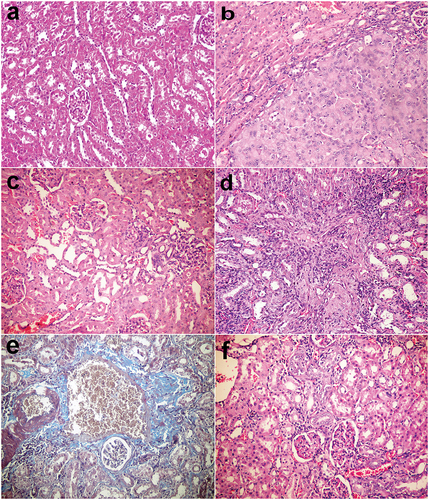
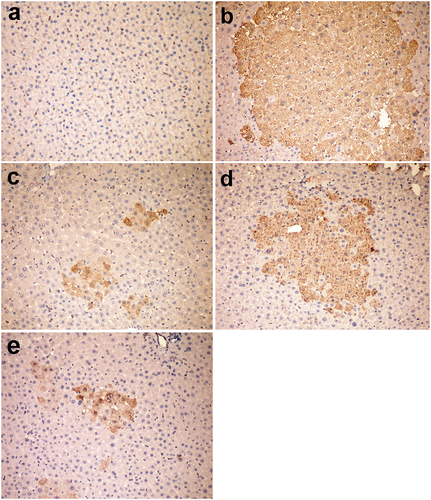
Figure 4. Liver, rat (38th week). (a) Negative staining for P-GST in group I (control group). (b) Large placental glutathione-S-transferase (P-GST) positive focus in group V injected with DENA. (c) Small-sized GST-P positive foci in group VI injected with DENA and treated with camel milk. (d) The medium-sized focus in group VII injected with DENA and treated with cisplatin. (e) Individual GST-P positive cells in group VI injected with DENA and treated with camel milk and cisplatin. Immunoperoxidase for GST-P 200 × (avidin–biotin–peroxidase complex method, Mayer’s Haematoxylin counterstain).