Figures & data
Figure 1. Effects of oral Cajanus cajan leaf extracts on body weight (g). (A) Kunming mice treated with WEC, EEC, BEC or DEC in acute toxicity test. Data are means ± SD (N = 20; half male and half female). (B) Male Sprague Dawley (SD) rat treated with WEC in sub-chronic toxicity, (C) female SD rat treated with WEC in sub-chronic toxicity, (D) male SD rat treated with EEC in sub-chronic toxicity, (E) female SD rat treated with EEC in sub-chronic toxicity. Data are means ± SD (N = 10 rats/group/sex in 4-week treatment period; N = 5 rats/group/sex for 2-week recovery period). *p < 0.05 vs. control.
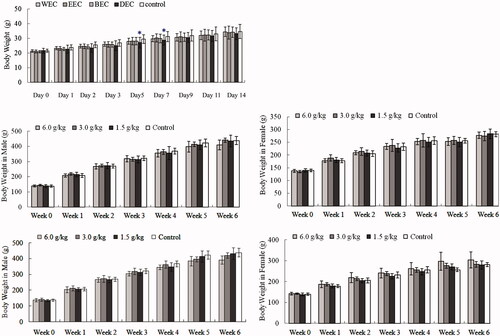
Table 1. Animal weight and relative weight gain (RWG) of Kunming mice before and after administration with the maximal oral dose of Cajanus cajan leaf extract or vehicle: acute toxicity assessment.
Table 2. Animal weight and relative weight gain (RWG) of SD rats treated with WEC, EEC or vehicle after a 4-week treatment period and a 2-week recovery period: sub-acute toxicity.
Figure 2. Effect of oral WEC on relative SD rat organ weight (g): sub-chronic toxicity. Data are means ± SD (N = 10; 5 rats/group/sex for 4-week treatment and 2-week recovery period), *p < 0.05 vs. control.
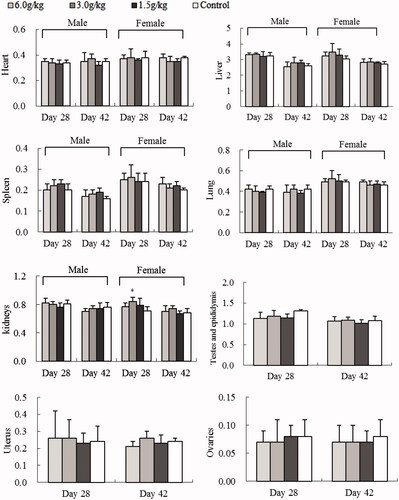
Figure 3. Effect of oral EEC on relative SD rat organ weight (g): sub-chronic toxicity. Data are means ± SD (N = 10; 5 rats/group/sex for 4-week treatment and 2-week recovery period), *p < 0.05, ** p < 0.01, ***p < 0.001 vs. control.
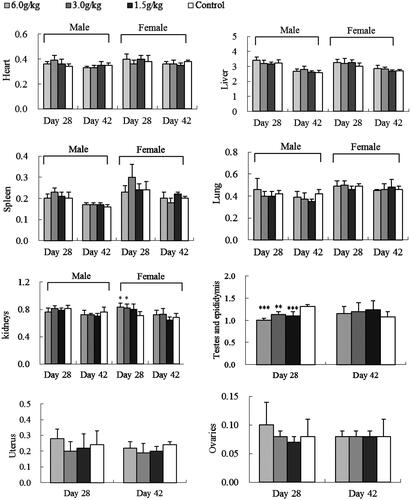
Figure 4. HPLC chromatograms of Cajanus cajan leaf extracts and reference compounds. (A) WEC, (B) BEC, (D) EEC, (E) DEC, (C) mixed water-soluble reference compounds, (F) mixed lipophilic reference compounds.1. Orientin, 2. Vitexin, 3. Genistin, 4. Pinostrobin, 5. Longistyline A, 6. Longistyline C.
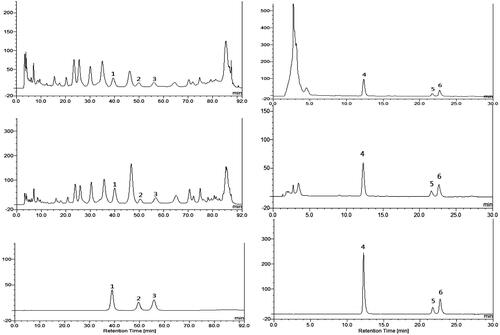
Table 3. HPLC assay for flavonoid and stilbenoid marker compounds in Cajanus cajan leaf extract (mg/g).
