Figures & data
Figure 2. Isolated peptide extracts from Lentinus squarrosulus. (a) Peptide elution profile obtained from Sephadex G-25 gel filtration chromatography. The rectangular box indicates the highest peptide containing fraction (F1) which was used for further evaluation. (b) SDS-PAGE of F1 fraction of gel filtration chromatography.
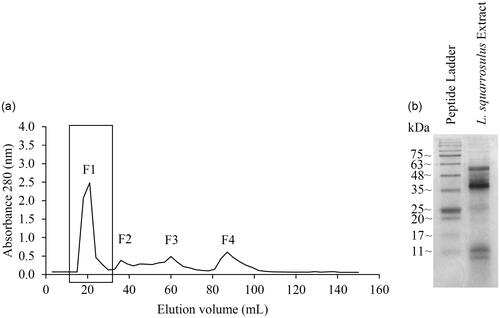
Figure 3. Apoptosis induction of peptide extracts from Lentinus squarrosulus. Cell viability of H460 lung cancer cells was evaluated after treatment with the extracts in (a) dose-dependent and (d) time-dependent manner. (b) There was a significant augmentation of apoptotic cell death in the incubation of peptide extracts at 5–40 μg/mL for 24 h. (e) Additionally, the effect of peptide extracts on apoptosis induction was early notified at 12 h of treatment of lung cancer cells with 20 μg/mL. (c and f) Apoptosis and necrosis cells were presented via co-staining of Hoechst33342 and propidium iodide. Values are means of the independent triplicate experiments ± SD. *p ≤ 0.05 versus non-treated control.
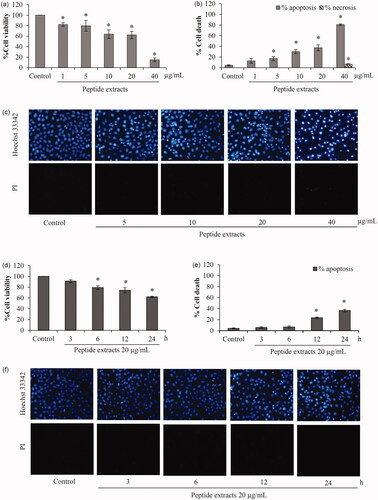
Figure 4. The apoptosis cells death was confirmed by the alteration of caspase-3 and PERP proteins. (a) Western blot analysis indicated an increase of active caspase-3 (cleaved- caspase-3) in peptide extracts-treated H460 cells. (b) As a substrate of activated caspase-3, the significant reduction of PARP was associated with the level of cleaved-caspase-3. Values are means of the independent triplicate experiments ± SD. *p ≤ 0.05 versus non-treated control.
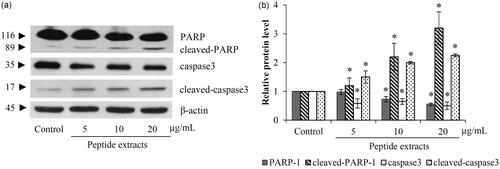
Figure 5. Peptide extracts stimulated both mitochondrial and death-receptor apoptotic pathway. (a and b) There were the reduction of Bcl-2 and up-regulation of BAX after treatment of lung cancer cells with 10–20 μg/mL of peptide extracts form Lentinus squarrosulus. (c and d) Protein-relating death-receptor pathway, c-FLIP and caspase-8 were decreased in peptide extracts-incubated H460 cells. Values are means of the independent triplicate experiments ± SD. *p ≤ 0.05 versus non-treated control.
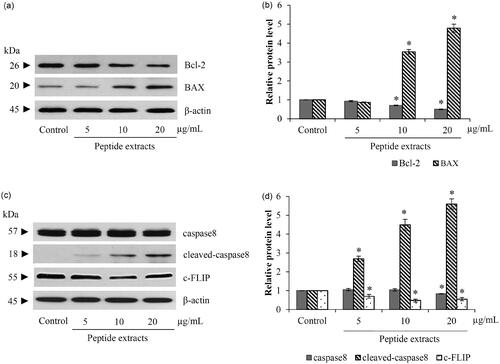
Figure 6. Selective anticancer activity of peptide extracts from Lentinus squarrosulus. (a) Low toxicity of the peptide extracts to non-cancer cells was indicated in human dermal papilla DPCs cells treated with 1–40 μg/mL peptides for 24 h. (b and c) Peptide extracts from L. squarrosulus induced cell death in various lung cancer cells. The anticancer activity of the extracts was also evaluated in H292 and H23 lung cancer cells. Values are means of the independent triplicate experiments ± SD. *p ≤ 0.05 versus non-treated control.
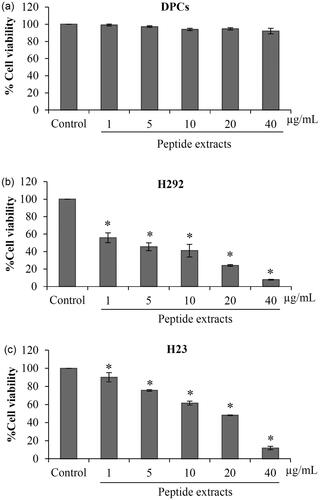
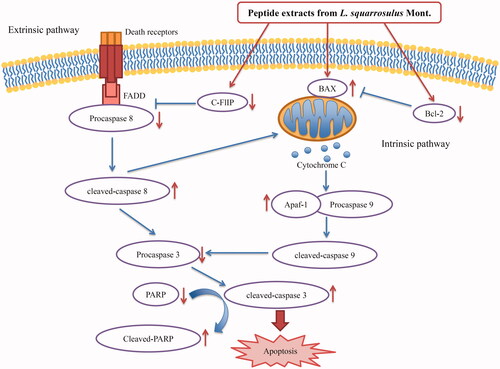
Figure 7. Proposed mechanistic scheme of anticancer activity of peptide extracts from Lentinus squarrosulus in human lung cancer cells. The extracts mediated a mitochondrial or intrinsic apoptotic pathway through the reduction of Bcl-2 and increase of BAX. Meanwhile, the extracts also decrease c-FLIP, an inhibitor for death-receptor regulating apoptosis.