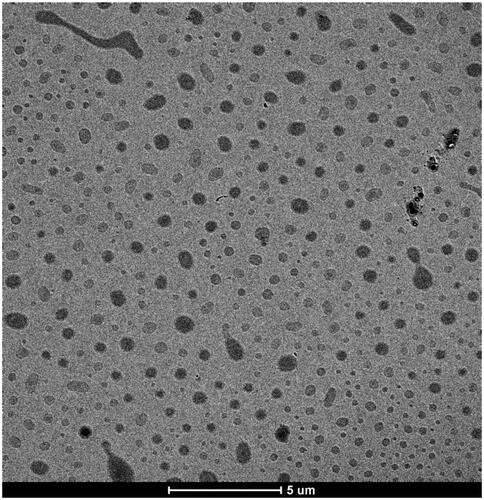
Figures & data
Table 1. Size, PDI and zeta potential values of Pluchea indica leaf extract NPs after storage in deionized water at 4°C for 4 weeks.
Figure 2. Effect of Pluchea indica leaf extract and P. indica leaf extract NPs on viability and proliferation of HO-1-N-1 cells. The cells were treated with the indicated concentration of Pluchea indica leaf extract or Pluchea indica leaf extract NPs for 2 h, followed by incubation in a serum free medium for (A) 24 h, (B) 48 h and (C) 72 h.
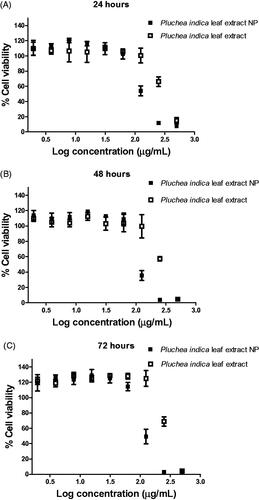
Figure 3. Migration of cells in serum free medium in the absence or presence of (A) 62.5 μg/mL and (B) 125 μg/mL Pluchea indica leaf extract and Pluchea indica leaf extract NPs. *p < 0.05 indicates a statistically significant difference between untreated and extract or NP treated cells.
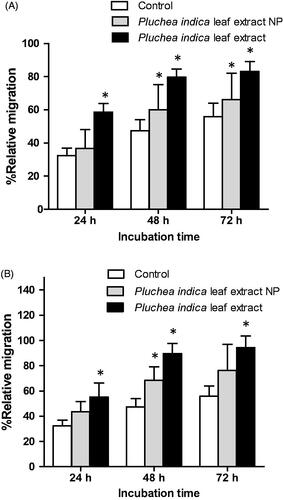
Figure 4. Micrographs showing the coverage of scratched wounds by HO-1-N-1 in the absence or presence of Pluchea indica leaf extract and Pluchea indica leaf extract NPs. (A) Negative control (serum free medium); (B) Pluchea indica leaf extract at 62.5 μg/mL; and (C) Pluchea indica leaf extract NPs at 62.5 μg/mL. The dash lines marked the boundaries of the scratched wounds.
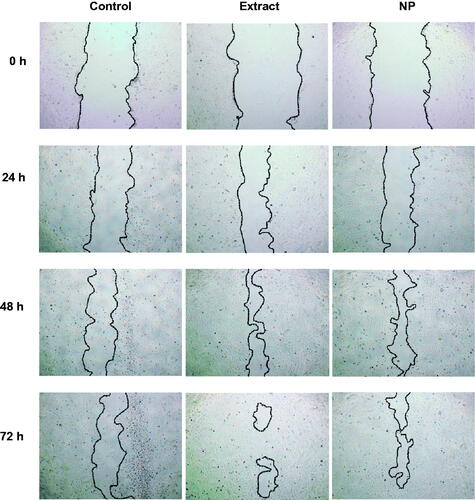
Figure 5. Characterization of unprocessed particles in Pluchea indica leaf extract and Pluchea indica leaf extract NPs in oral spray formulation after tested accelerated stability test of heating–cooling cycles for six cycles. (A) Hydrodynamic diameter, (B) PDI, (C) pH of oral spray formulation.
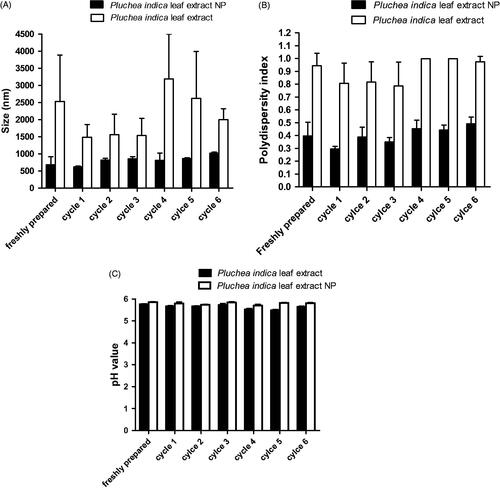
Table 2. Size, PDI and pH of Pluchea indica leaf extract NPs before and after storage in oral spray formulation at room temperature, 4 °C and 45 °C for 30 days.