Figures & data
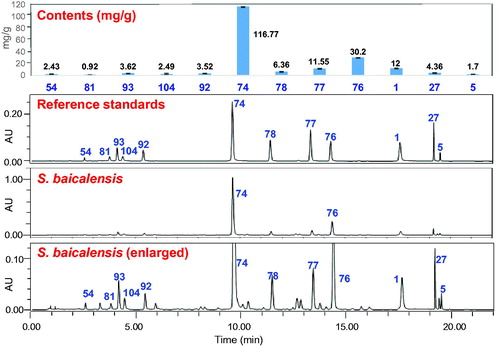
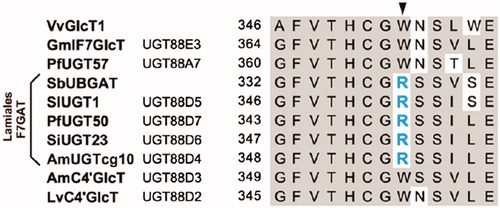
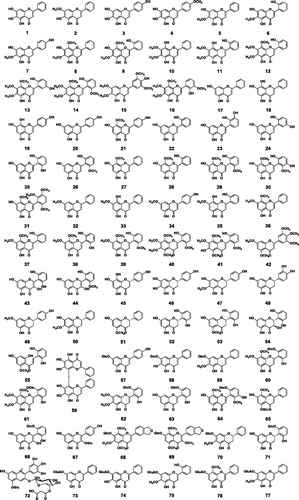
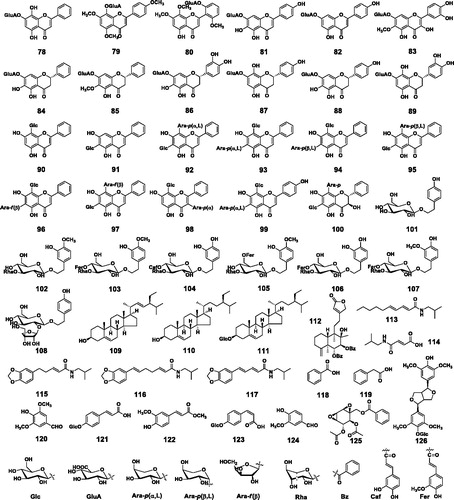
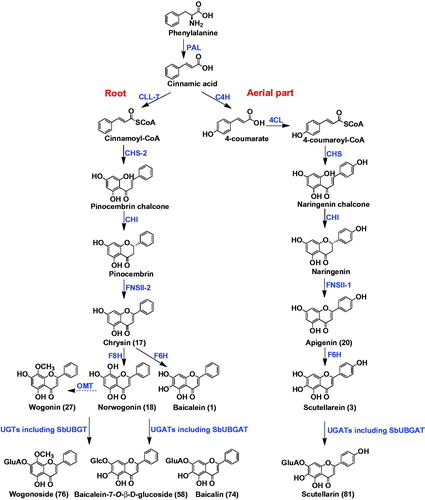
Table 1. Compounds 1-126 from Scutellaria baicalensis.
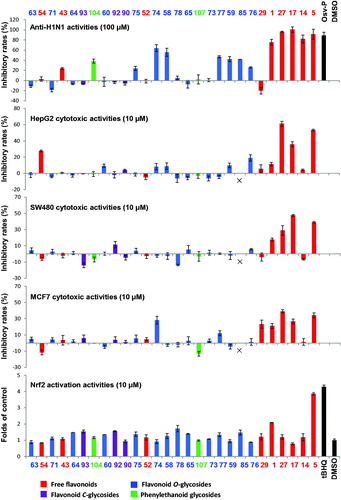
Table 2. The anti-tumor activities of Scutellaria baicalensis and its compounds.
Table 3. The neuroprotective activities of Scutellaria baicalensis and its compounds.