Figures & data
Table 1. Comparison of various types of liver injury. (A summary and comparison of all kind of major models experimental models of liver injury, including type, model, key index, molecular mechanism, advantages, and disadvantages.)
Table 2. A summary of the effect of traditional Chinese medicine on liver injury. (This is an overview of liver injury treated by Chinese medicine by the end of May 2018, including the name of herb or compound, parts or solvent, liver injury model, mechanism of action, and reference.)
Figure 1. CCl4-induced liver injury. CCl4 trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation, macrophages activation, mitochondrion path, and oxidative stress, resulting in apoptosis, hepatic fibrosis, and liver injury. COX-2: epoxide hydrolase; PGs: prostaglandins; HSC: hepatic stellate cells; CYP450: cytochrome P450; TNF-α: tumor necrosis factor α; IL: interleukin; NF-κB: nuclear factor κB; AP-1: activated protein transcription factor 1; PLA2: phosphatase A2; TGF-β: transforming growth factor β; Cyt-C: cytochrome C.
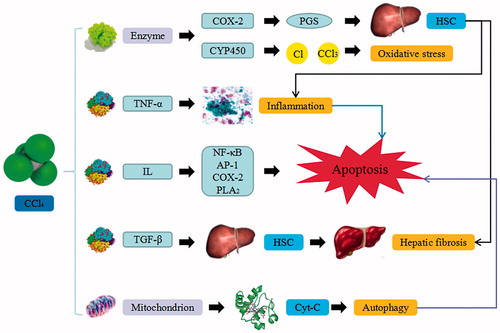
Figure 2. d-GalN-induced liver injury. d-GalN trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation, metabolic disorder, and oxidative stress, resulting in necrosis, apoptosis, autophagy, and liver injury. UDP: uridine diphosphate; UTP: uridine triphosphate; UDPase: UDP-glucose pyrophosphorylase; GSH: glutathione; TNF-α: tumor necrosis factor α.
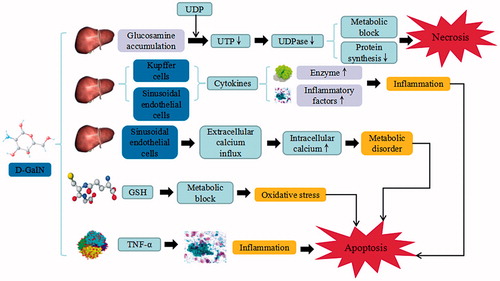
Figure 3. ANIT-induced liver injury. ANIT trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation, hepatotoxicity, cholestatic jaundice, and hyperbilirubinemia, resulting in apoptosis, autophagy, cholestasis, and liver injury. GSH: glutathione; IL: interleukin; MIP-2: macrophage inflammatory protein-2; NADPH: nicotinamide adenine dinucleotide phosphate.
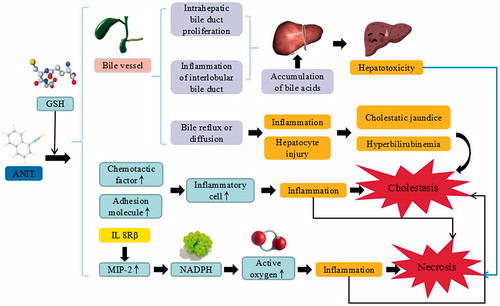
Figure 4. DMN-induced liver injury. DMN trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation, and activation of HSC, resulting in apoptosis, autophagy, sedimentation of ECM, hepatic fibrosis, and liver injury. CYP2EI: cytochrome P2EI; TNF-α: tumor necrosis factor α; IL: interleukin; α-SMA: α smooth muscle actin; TGF-β: transforming growth factor β; CTGF: connective tissue growth factor; TIMP1: tissue inhibitor of metalloproteinases 1; Smad: drosophila mothers against decapentaplegic protein; ECM: extracellular matrix.
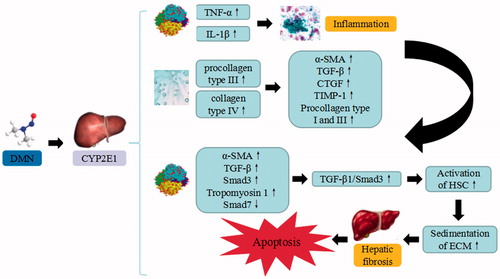
Figure 5. TAA-induced liver injury. TAA trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation, metabolic disorder, enterogenous toxicemia, and oxidative stress, resulting in hepatic necrosis, apoptosis, and liver injury. CYP450: cytochrome P450; IETM: intestinal endotoxemia; iNOS: inducible nitric oxide synthase; eNOS: endothelial nitric oxide synthase; TXA2: thromboxane A2; ROS: reactive oxygen species.
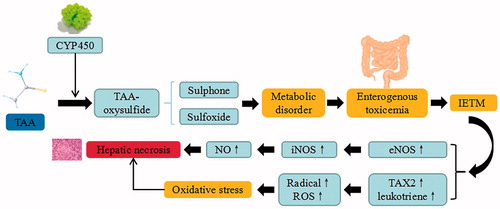
Figure 6. ConA-induced liver injury. ConA triggers the continuous development of hepatocyte injury by inducing cell stress via inflammation and macrophages activation, resulting in hepatic necrosis and liver injury. NF-κB: nuclear factor κB; Irf-3: Interferon regulatory factor -3; NLRP3: NACHT, LRR and PYD domains-containing protein 3; TNF-α: tumor necrosis factor α; IFN-γ: Interferon-γ; IL: interleukin.
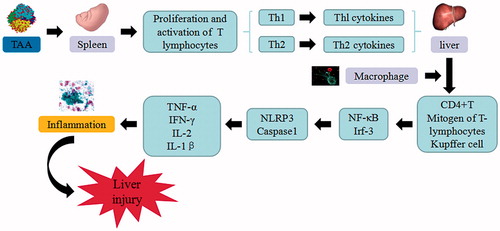
Figure 7. BCG/LPS-induced liver injury. BCG/LPS trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation and sensitization, resulting in hepatic necrosis and liver injury. TNF-α: tumor necrosis factor α; IL: interleukin.
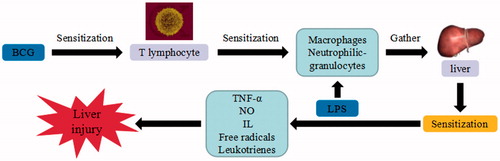
Figure 8. d-GalN/LPS-induced liver injury. d-GalN/LPS triggers the continuous development of hepatocyte injury by inducing cell stress via inflammation, metabolic block, protein synthesis decrease, and hepatocyte DNA fracture and degradation, resulting in hepatic necrosis and liver injury. UDP: uridine diphosphate; UTP: uridine triphosphate; TLR: toll-like receptor; TNF-α: tumor necrosis factor α; IL: interleukin.
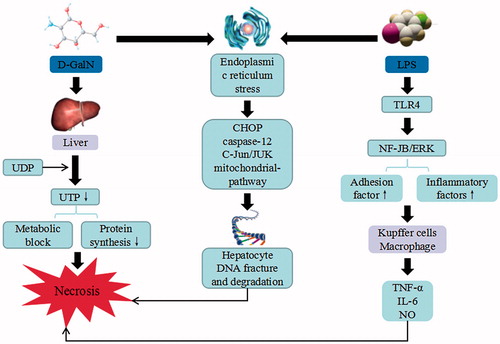
Figure 9. Alcoholic-induced liver injury. Alcohol triggers the continuous development of hepatocyte injury by inducing cell stress via inflammation, oxidative stress, protein synthesis decrease, and hepatocyte DNA fracture and degradation, resulting in hepatic necrosis, apoptosis, hepatic fibrosis, and liver injury. ADH: alcohol dehydrogenase; ALDH: aldehyde dehydrogenase; CYP2EI: cytochrome P2EI; ROS: reactive oxygen species; Cyt-C: cytochrome C; RIP: receptor-interacting protein; TRADD: tumor necrosis factor receptor-related domain death protein; FADD: fas-associating protein with a novel death domain; TNF-α: tumor necrosis factor α; IL: interleukin; NF-κB: nuclear factor κB.
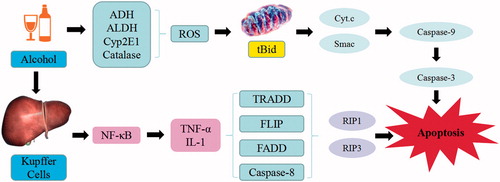
Figure 10. APAP-induced liver injury. APAP trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation, oxidative stress, and JNK pathway, resulting in hepatic necrosis, apoptosis, hepatic fibrosis, and liver injury. CYP450: cytochrome P450; NAPQI: N-acetyl-p-benzoquinonimine; GSH: glutathione; ROS: reactive oxygen species; iNOS: inducible nitric oxide synthase; NF-κB: nuclear factor κB; TNF-α: tumor necrosis factor α.
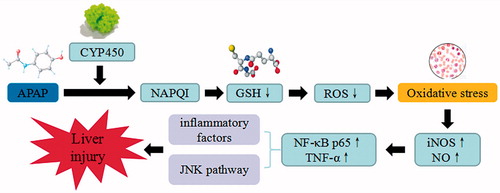
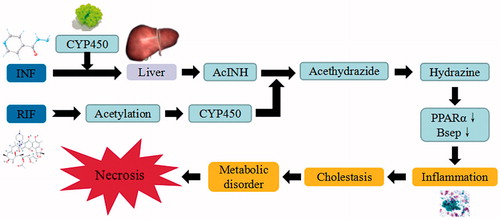
Figure 11. INH/RIF-induced liver injury. INH/RIF trigger the continuous development of hepatocyte injury by inducing cell stress via inflammation, cholestasis, and metabolic disorder, resulting in hepatic necrosis, apoptosis, and liver injury. CYP450: cytochrome P450; PPAR: peroxisome proliferator-activated receptors.