Figures & data
Figure 1. Experimental protocol. After 10 min of acute perfusion with a tested substance (ACSF, 0.01% DMSO, 10 µg/mL ECa 233 in 0.01% DMSO, or 100 µg/mL ECa 233 in 0.01% DMSO), an I-O curve was recorded (the stimulus intensities were varied from 30 μA to 300 μA). Then, the stimulus intensity of half-maximal fEPSP amplitude from the I-O curve was used for the stimulation on the hippocampal slices throughout the experiment. The baseline activity was recorded for 20 min. Then, the HFS was delivered at the same stimulus intensity to evoke LTP, and the activity was continually recorded for 60 min (LTP phase). After 60 min of recording, the solutions were reverted to ACSF, and the activity was continually recorded for 15 min (washed-out phase).
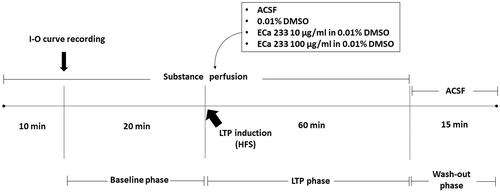
Figure 2. Acute effect of ECa 233 on the hippocampal basal synaptic transmission. The I-O curve represents the basal synaptic transmission. The stimulus intensities varying from 30 μA to 300 μA were applied at the Schaffer collateral pathway. The fEPSP slopes were recorded at the dendritic layer of the CA1 subregion. Data are presented as mean ± SEM (n = 4).
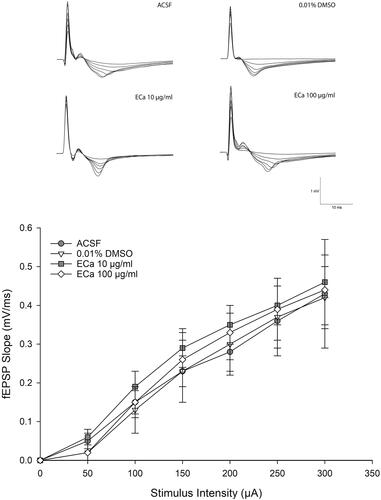
Figure 3. Enhancement of hippocampal long-term potentiation magnitude after acute treatment with different ECa 233 concentrations. Data are presented as mean ± SEM (n = 4). ap = 0.041 compared with the ECa 100 µg/mL group; bp = 0.037 compared with the ACSF group; and cp = 0.008 compared with the 0.01% DMSO group.
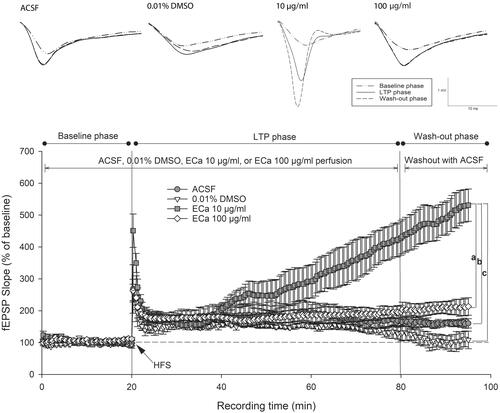
Figure 4. Acute effect of ECa 233 on the hippocampal long-term potentiation. The percentage of the baseline fEPSP slope at the last 10 min at each phase was averaged. Data are presented as mean ± SEM (n = 4). *p = 0.000 compared with the baseline phase; #p = 0.000 compared with the LTP phase.
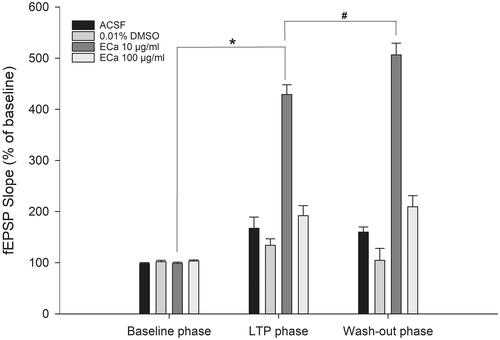
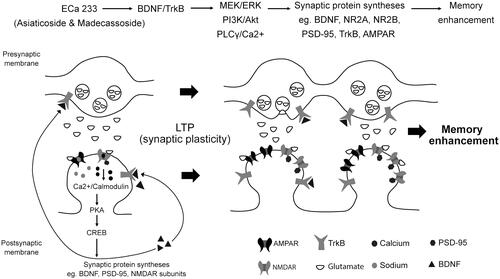
Figure 5. Schematic representation of the possible mechanisms of ECa 233 on synaptic plasticity. Low doses of ECa 233 via the main effects of asiaticoside and madecassoside might activate the MAPK/ERK, PI3K/AKT, and PLCγ pathways, leading to many protein syntheses, including BDNF, NMDAR subunits (NR2A and NR2B), AMPAR, PSD-95, TrkA, TrkB, and synapsin I. Moreover, ECa 233 might promote the phosphorylation activity of p-CaMKII, p-PKACβ, and p-CREB. Then, BDNF binds to TrkB at both pre- and postsynaptic membranes. These bindings trigger three signalling cascades (PLCγ/Ca2+, PI3K/Akt, and MEK/ERK) mediating synaptic transmission (glutamate release elevation), LTP, and neurite outgrowth. The enhanced expression of plasticity-related proteins mediates the enhancement of synaptic plasticity, which is exhibited as the hippocampal LTP. Finally, all cellular changes that are part of the basic mechanism of learning and memory might be expressed as memory enhancement.