Figures & data
Figure 1. Study flow chart. SAD: Single ascending-dose, once daily; MAD: Multiple ascending-dose, three times daily; CALAS: Capsule of alkaloids from leaf of A. scholaris. The further tests of cohort 7 (600 mg) and 8 (720 mg) cohorts in SAD study were not conducted due to the occurrence of adverse reactions in cohort 6 (480 mg).
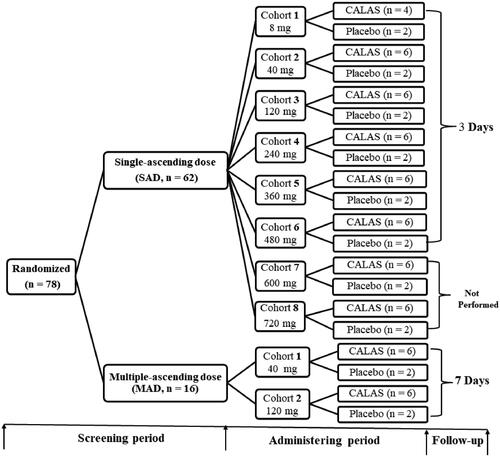
Table 1. Abnormal laboratory parameters after single ascending-dose administration.
Table 2. Abnormal laboratory parameters after multiple ascending-dose administration.
Table 3. Adverse events profile with a single ascending-dose of CALAS.
Table 4. Adverse events profile with a multiple ascending-dose of CALAS.
Table 5. Adverse events with CALAS in grades.
Table 6. Adverse drug reactions profile with a single ascending-dose of CALAS.
Table 7. Adverse drug reactions profile with a multiple ascending-dose of CALAS.
Table 8. Adverse drug reactions in grades.
