Figures & data
Figure 3. SRM chromatograms of thonningianin A and IS in a blank plasma sample (A), a blank sample spiked the analyte (LLOQ) and IS (B), and a plasma sample at 0.083 h after oral administration of 20 mg/kg thonningianin A (C).
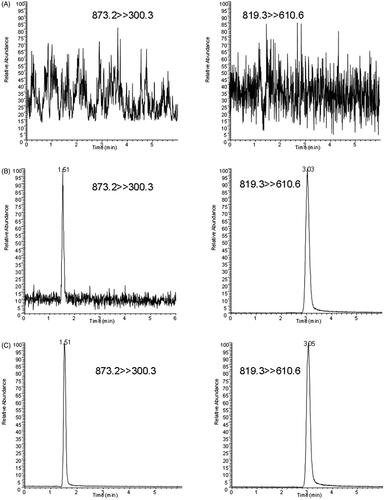
Table 1. Precision and accuracy of the LC-MS/MS method (n = 5).
Table 2. Stability of the thonningianin A in rat plasma (n = 5).
Table 3. Recovery and matrix effect of the thonningianin A and IS in rat plasma (n = 5).
Figure 4. Mean plasma concentrations of thonningianin A after oral administration to rats orally (p.o., 10, 20, and 40 mg/kg).
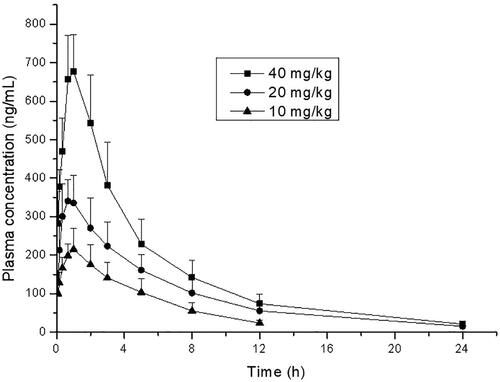
Table 4. Mean pharmacokinetic parameters of thonningianin A after oral administration to rats orally.